Faculty spotlight: Professor Alena Talkachova

December 2019—A heart’s mechanical contractions are triggered by electrical waves of excitation propagating through cardiac tissue. Abnormalities in these waves, including fast and chaotic propagation in the heart, may facilitate cardiac arrhythmias, such as ventricular tachycardia (VT), ventricular fibrillation (VF), and atrial fibrillation (AF), which can lead to sudden cardiac death. My laboratory investigates the electrical activity of the whole heart from a nonlinear dynamics perspective, to better understand why and how such arrhythmias develop in the heart.
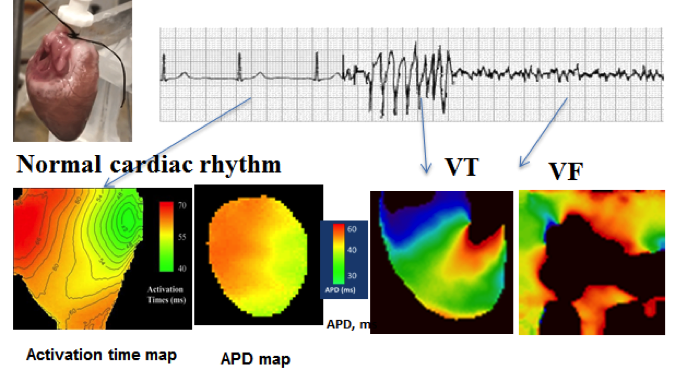
To do this, we use high-resolution optical mapping techniques to visualize electrical and/or calcium activity and acquire basic electrophysiological properties of healthy and diseased hearts during normal cardiac rhythms as well as arrhythmias (see Figure). Then, we analyze the complex spatio-temporal dynamics of the heart’s electrical and mechanical activity to reveal the mechanisms leading to arrhythmias. This allows us to develop approaches to prevent and control arrhythmias, and therefore reduce sudden cardiac death.
For instance, we recently developed several novel approaches to help doctors identify potentially dangerous sites when performing ablation therapy in patients with persistent AF. AF is the most common sustained cardiac arrhythmia in humans and a prognostic marker for stroke, heart failure, and even death. It’s believed that AF is maintained via rapid reentry sources (rotors), making it very important to accurately identify the location of the rotors’ pivot points. These pivot points are the primary target for clinical ablation therapy. We’ve developed several novel signal analysis approaches for identifying these pivot points, which we’ve validated using optical mapping experiments in ex-vivo rabbit hearts. Applying these techniques in clinical settings could create a future where there are personalized approaches for successfully diagnosing and treating arrhythmias.
Our basic science, therapeutic, and translational research is supported by external NSF and NIH funds, internal Institute for Engineering (IEM) support, as well as collaborative efforts with LivaNova, Boston Scientific, and Medtronic.