The Hoye group discloses “Radial hexadehydro-Diels-Alder reactions”

MINNEAPOLIS / ST. PAUL (10/18/2021)—Dan Lee, a 5th year graduate student in Professor Thomas Hoye’s research group, discloses a new synthetic strategy enabled by the hexadehydro-Diels-Alder (HDDA) reaction. This research article, “Radial Hexadehydro-Diels-Alder Reactions” has been published in Chem. [doi:10.1016/j.chempr.2021.08.010]
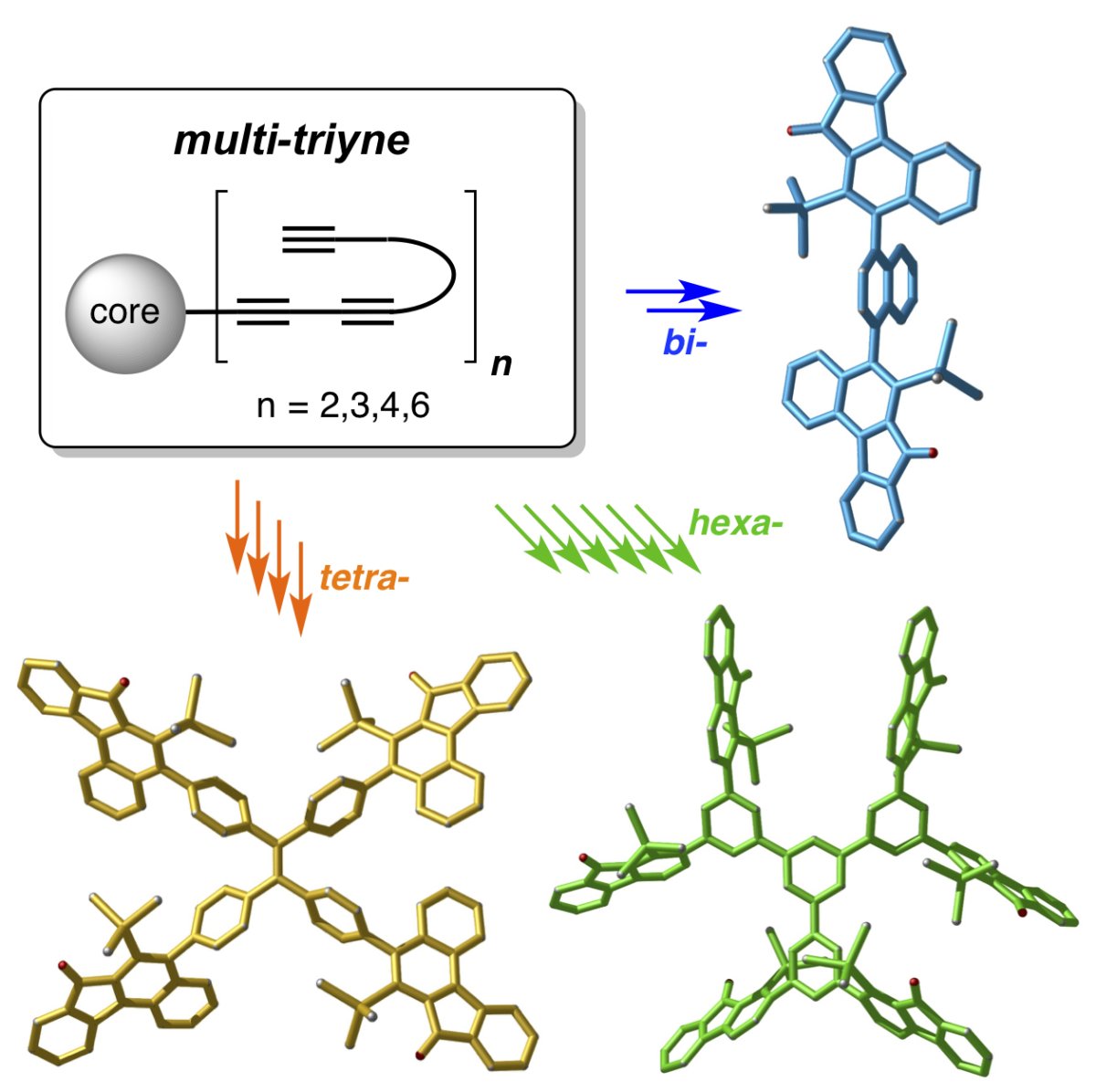
In this work, Hoye and co-workers describe HDDA reactions in which the substrates are designer multi-ynes arrayed upon a common, central template. These undergo sequential, multiple cycloisomerization (termed radial-HDDA) reactions to produce architecturally novel polycyclic compounds in a single operation. Diverse product topologies are accessible, ranging from highly fused, polycyclic aromatic compounds (PACs) to architectures having structurally complex arms adorning central phenylene or expanded phenylene cores. The late-stage and de novo creation of multiple arenes in these multi-benzyne processes constitutes a fundamentally new synthetic strategy for constructing novel molecular topologies.