Past Seminars & Events
Professor Phillip Milner
Tuesday, Oct. 31, 2023, 9:45 a.m. through Tuesday, Oct. 31, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Phillip Milner
Department of Chemistry & Chemical Biology
Cornell University
Abstract
Simplifying Synthesis at the Interface of Organic and Materials Chemistry
Porous framework materials, including metal- organic frameworks (MOFs), are highly tunable materials with myriad potential applications ranging from chemical separations to gas storage to catalysis. This is due to the unusual local environment offered by their pores. Herein we will discuss how this tunability can be used to unlock new reactive species relevant to organic synthesis and catalysis, focusing on fluorination chemistry, which is critical to the pharmaceutical, polymer, and agrochemical industries. We will also draw inspiration from organic chemistry for the design of new chemical separations and electrocatalytically active materials.
Phillip Milner
Phill was born a stone’s throw from Ithaca in Towanda, PA and grew up near Rochester, NY. Phill attended Hamilton College near Utica, NY, where his love of synthetic organic chemistry was born while working on radical cyclizations with Prof. Ian Rosenstein. Phill graduated from Hamilton College in 2010 with B.A.s in Chemistry and Mathematics, and went on to pursue his Ph.D. in Chemistry with Prof. Stephen Buchwald at the Massachusetts Institute of Technology (MIT). In the Buchwald group, Phill carried out extensive mechanistic studies of the Pd-catalyzed fluorination of aryl (pseudo)halides, a reaction of importance due to the prevalence of aryl fluorides in pharmaceuticals and agrochemicals. Phill also developed the nearly instantaneous 11C-cyanation of aryl halides for the synthesis of PET radiotracers. Phill joined the group of Prof. Jeffrey Long at the University of California, Berkeley upon graduating from MIT in 2015. As a post-doctoral Fellow in the Long group, Phill designed amine-functionalized metal–organic frameworks for the removal of CO2 from the flue gas emissions of power plants. In 2018, Phill joined the Department of Chemistry and Chemical Biology at Cornell University, where his research is focused broadly at the intersection of organic, inorganic, and materials chemistry. Phill is a member of the Cornell Center for Materials Research (CCMR) and the Cornell Energy Systems Institute (CESI), a Cornell Atkinson Center for Sustainability Faculty Fellow, and a field member in the Department of Chemical and Biomolecular Engineering. Phill’s independent awards and honors include: Camille Dreyfus Teacher-Scholar Award (2023), NSF CAREER Award (2021), Robert A. and Donna B. Paul Award for Excellence in Advising (2021), Scialog Fellowship (2020), Department of Energy Early Career Award (2020), and NIH Maximizing Investigator’s Research Award (2020).
Hosted by Professor Courtney Roberts
Professor Aleksandr V. Zhukhovitskiy
Tuesday, Oct. 24, 2023, 9:45 a.m. through Tuesday, Oct. 24, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Aleksandr (Alex) V. Zhukhovitskiy
William R. Kenan Jr. Faculty Fellow
Department of Chemistry
University of North Carolina-Chapel Hill
Abstract
Advancing the logic of polymer synthesis, modification, and degradation
The polymer backbone is fundamental to the polymer’s identity and properties. My seminar will focus on the development of metathesis mechanisms to access heteroatom-rich polymer backbones, new editing tools to transform existing polymer backbones into different ones, and both strategies and tactics to depolymerize commodity polymeric materials into valuable small molecules. Specifically, I will discuss iridium-guanidinate catalyzed ring-opening metathesis of cyclic carbodiimides and the current directions toward diazene metathesis, as well as an array of rearrangement transformations—including Ireland-Claisen and aza-Cope—applied to edit the backbones of polymers. Besides the focus on polymer backbones, retrosynthetic logic applied to polymeric materials will be another common thread woven throughout this seminar, as it is a central element of the research in the Zhukhovitskiy group.
Alex Zhukhovitskiy
The Zhukhovitskiy Group at the University of North Carolina is focused on fundamental methods development as related to polymer chemistry. They blend polymer science, organometallic chemistry, and mechanistic studies to uncover new strategies for the synthesis of polymers such as carbodiimide ring-opening metathesis polymerization sigmatropic rearrangements as a strategy for polymer skeletal editing.
Hosted by Professor Ian Tonks
Professor Titel Jurca
Friday, Oct. 20, 2023, 4 p.m. through Friday, Oct. 20, 2023, 5:30 p.m.
331 Smith Hall
Zoom Link
Professor Titel Jurca
Department of Chemistry
University of Central Florida
Abstract
Building a Toolbox for Sustainable Synthesis and Catalysis
Our multidisciplinary research group focuses on molecular inorganic synthesis, thin film materials via atomic layer deposition (ALD), and heterogeneous catalysis for fine chemical transformations. Our goal is the convergence of these subdisciplines of inorganic chemistry towards the synthesis of complex hierarchical catalyst systems that are active, selective, and highly reusable. Specifically, we are seeking to discover and develop sustainable synthetic methodologies enroute to our desired end products – often these alternative routes enable the synthesis of previously inaccessible, or difficult to access products. To that end, the presentation will focus on three of our current areas of interest:
- Mechanochemical synthesis: by leveraging mechanochemical forces via vibratory ball milling or ultrasonic irradiation, we can expedite the synthesis of Schiff base coordination complexes. These reactions can be performed solvent-free or solvent-minimal and facilitate the formation of target compounds in one-pot and one- step from multiple starting materials in minutes-to-hours compared to conventional multi-day, multi-step processes.
- Silane-based reductions: using stoichiometric silanes, high- valent, mid d-block metal halides can be stoichiometrically reduced to highly reactive mid-valent synthons (e.g. MoCl 3 from MoCl 5 ). The reactions are facile and produce only H 2 and recoverable and reusable chlorosilanes as byproduct. The resulting mid-valent metal chlorides form ideal starting points towards new precursors for ALD and chemical vapor deposition (CVD); enabling technologies for coatings, electronic materials, and heterogeneous catalysis.
- Monolith-based nanocatalysts: controlled growth of nanocatalysts on contiguous Ni foams; porous, contiguous membranes that enable facile handling, reuse, and direct applicability to flow reactions. The application of these materials towards the catalytic hydrogenation of nitro, alkene, and alkyne moieties, in batch and flow, under mild conditions will be discussed.
Titel Jurca
Originally from Romania, Titel emigrated to, and grew up in Ottawa Canada. He received his BSc in 2008 from the University of Ottawa, where he worked with Deryn Fogg on high throughput screening of metathesis catalysts. In the interim, he spent a summer in the lab of Doug Stephan at the University of Windsor working on FLP chemistry. He then returned to the University of Ottawa to pursue a PhD in main group coordination chemistry with Darrin Richeson (2012). He followed this with a Marie Curie postdoctoral fellowship at the University of Bristol with Ian Manners (2012-15) working on main group polymers and a second postdoctoral fellowship at Northwestern University with Tobin Marks (2015- 2017) working on ALD precursors and supported catalysts. In 2017 he began his independent career at the University of Central Florida.
Website: jurcalab.com
Hosted by Professor Gwendolyn Bailey
Professor Lingjun Li
Thursday, Oct. 19, 2023, 9:45 a.m. through Thursday, Oct. 19, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Lingjun Li
School of Pharmacy and Department of Chemistry
University of Wisconsin-Madison
Abstract
Advancing Biomedical Research via Innovation in Mass Spectrometry (MS)-based Approaches
Comprehensive characterization of all signaling molecules in a biological system with chemical, spatial and temporal information is often critical to deciphering their functional roles, yet it poses a daunting challenge. In this presentation, I will present our recent progress on the development of a multi-faceted mass spectrometry (MS)-based analytical platform to probe neuronal signaling with enhanced sensitivity and selectivity. By combining chemical labeling, micro-scale separation, and tandem MS sequencing techniques, we discovered more than 300 novel neuropeptides in several model organisms. Moreover, both mass spectrometric imaging (MSI) technology and in vivo microdialysis sampling tools have been developed and implemented to follow neuropeptide distribution and secretion with unprecedented details. Additionally, several in situ chemical derivatization strategies have been developed to enable spatial mapping of various biomolecules including lipids and glycans in complex biological samples, such as human cell lines and cancer tissue samples. Recent progress towards single- cell lipidomics enabled by dual-polarity ionization and ion mobility MS imaging will be highlighted as well.
Furthermore, we are developing multiplexed isobaric and isotopic tagging strategies to discover, identify and evaluate candidate biomarkers of Alzheimer’s disease (AD) in cerebrospinal fluids (CSFs) obtained from asymptomatic cognitively-healthy middle- aged adults, older cognitively-normal adults, and patients with mild cognitive impairment (MCI) and AD. A large-scale comparative glycoproteomic analysis via the 12-plex DiLeu (N,N-dimethyl leucine) tagging strategy revealed distinct glycosylation patterns and dynamic changes of certain glycoforms in CSF samples collected from the control, MCI, and AD patients. Additionally, we report on a multiplexed quantitation method for simultaneous proteomics and amine metabolomics analyses via nanoflow reversed phase LC-MS/MS, exploiting mass defect-based DiLeu (mdDiLeu) labeling. Several on-going efforts and future perspectives provided by these enabling technologies will be highlighted and discussed.
Lingjun Li
Dr. Lingjun Li is a Vilas Distinguished Achievement Professor and the Charles Melbourne Johnson Distinguished Chair Professor of Pharmaceutical Sciences and Chemistry at the University of Wisconsin-Madison (UW-Madison). Dr. Li received her Ph.D. degree in Analytical Chemistry/ Biomolecular Chemistry from the University of Illinois at Urbana-Champaign in 2000. She then did joint postdoctoral research at the Pacific Northwest National Laboratory and Brandeis University before joining the faculty at UW-Madison in December 2002. Dr. Li’s research interests include the development of novel mass spectrometry (MS)- based tools such as new isotopic and isobaric labeling strategies that enable hyperplexing for quantitative proteomics, peptidomics, and glycomics, and their applications in neuroscience and cancer research. She and her team also develop microscale separations, in vivo microdialysis and imaging MS tools for functional discovery of neuropeptides in model organisms and (glyco)protein biomarkers in neurodegenerative diseases with a strong focus on Alzheimer’s disease. Her lab also explores novel use of ion mobility MS to address technical challenges in peptidomic research. Professor Li has established a highly productive research program and published more than 400 peer-reviewed research journal papers (with H-index of 64, and more than 15,340 citations) and has given more than 300 invited talks. Dr. Li is passionate about training next generation of scientists and has successfully trained and graduated 65 PhDs and is currently training 25 PhD graduate students, 5 postdoctoral scientists, and 6 undergraduate students. Dr. Li has been recognized with numerous awards, including ASMS Research Award, NSF CAREER Award, Sloan Fellowship, PittCon Achievement Award, and ASMS Biemann Medal, and was named one of the Top 50 most influential women in the analytical sciences in 2016 and was recently featured in the 2019 and 2021 Top 100 Power List by the Analytical Scientist (on a global scale). Dr. Li is currently serving as an Associate Editor for the Journal of the American Society for Mass Spectrometry (JASMS) and sitting on the Advisory Board for Analytical and Bioanalytical Chemistry and Mass Spectrometry Reviews.
Hosted by Professor Varun Gadkari
Professor Christopher A. Alabi
Tuesday, Oct. 17, 2023, 9:45 a.m. through Tuesday, Oct. 17, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Christopher A. Alabi
Robert Frederick Smith School of Chemical and Biomolecular Engineering
Cornell University
Abstract
Sequence-defined macromolecules with tunable nonbonding sites
The precise placement of functional groups along a polymer chain plays a key role in encoding specific intra- and intermolecular interactions that direct self-assembly into discrete architectures. Biopolymers known to associate with specific geometries and stoichiometries have been exploited for the assembly of synthetic building blocks. However, such systems are neither scalable nor amenable to the relatively harsh conditions required by various materials science applications, particularly those involving non-aqueous environments. To overcome these limitations, I will discuss our work on sequence-defined oligocarbamates (SeDOCs) and data highlighting the effect of sequence on network topology, mechanical and optical properties. I will then discuss SeDOC ligands that assemble into duplexes through complementary hydrogen bonds between thymine (T) and diaminotriazine (D) pendant groups. We’ve successfully synthesized monovalent, divalent, and trivalent SeDOCs and characterized their hybridization via a variety of techniques, including diffusion ordered spectroscopy, 1 H-NMR titration, and isothermal titration calorimetry. Our findings reveal that the binding strength of monovalent oligomers with complementary pendant groups is entropically driven and independent of monomer sequence, that hybridization of multivalent oligomers is cooperative, and that SeDOCs ligands bearing D and T groups exhibit signatures of enthalpy- entropy compensation.
Christopher A. Alabi
Christopher Alabi holds a Bachelor of Science in Chemistry from New York University and a Bachelor of Engineering in Chemical Engineering from Stevens Institute of Technology. He completed his PhD in Materials Chemistry under the guidance of Mark Davis at the California Institute of Technology. In 2009, he pursued an NIH Postdoctoral fellowship at the Massachusetts Institute of Technology, working alongside Robert Langer and Dan Anderson. In 2013, he joined the Cornell faculty as an Assistant Professor in the School of Chemical and Biomolecular Engineering. His early research achievements include investigations into synthesis and properties of novel sequence-defined macromolecules, recognized through accolades such as the PhRMA Foundation Research Starter Award, NSF CAREER Award, Cornell Engineering Research Excellence Award (2016), and the PMSE Young Investigator Award. Acknowledging his commitment to teaching and mentorship, he received the 2017 Tau Beta Pi Professor of the Year Award and, more recently, the 2022 Richard F. Tucker Excellence in Teaching Award. Research conducted in the Alabi lab is focused on deciphering how the composition and sequence of macromolecular chains influence their chemical, structural, and biological attributes, with the ultimate aim of engineering sustainable functional materials and biomolecular therapeutics.
Hosted by Professor Theresa Reineke
Professor Balyn Zaro
Thursday, Oct. 12, 2023, 9:45 a.m. through Thursday, Oct. 12, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Balyn Zaro
Department of Pharmaceutical Chemistry
University of California, San Francisco
Abstract
A Chemical Biology Approach to Innate Immunity
Macrophages regulate how the immune system recognizes self. When a foreign/exhausted cell or pathogen is detected, macrophages engulf and destroy it in a process called phagocytosis. Anti-phagocytic signaling axes, also referred to as ‘don’t eat me’ (DEM) signals, exist between macrophages and other cells. To evade macrophages, healthy cells express DEM signal ligands on their surface. When a DEM ligand engages a DEM receptor on a macrophage, downstream signaling blocks phagocytosis. Four DEM signal ligand- receptor pairs have been discovered, and it is hypothesized that there are others. Dysregulation of DEM signaling has been implicated in cancer, infectious disease, neurodegeneration, and atherosclerosis. In addition to DEM signal ligands, mammalian cells also present ‘eat me’ (EM) ligands, which engage macrophage EM receptors, and are required for phagocytosis. Despite the fundamental role these signals play in basic and disease biology, relatively little is known about the biological mechanisms and consequences of these pathways. Learning to harness and exploit DEM and EM signaling would result in a wave of new biologic discovery in human health and disease. However, highly-selective chemical probes and new specialized chemical proteomic approaches are required. To this end, my lab has developed selective chemical tools and novel proteomic techniques to study macrophage function and host-pathogen interactions. Our new toolkit has lead to discoveries in macrophage function that reveal how we combat disease and may change the way we design immunotherapeutics.
Balyn Zaro
Balyn Zaro, PhD, grew up in California and Connecticut. As an undergraduate researcher at University of Southern California (USC) she studied synthetic methodology and organic chemistry with G.K. Surya Prakash and Nobel laureate George A. Olah. She remained at USC for her PhD studies in chemical biology as the first graduate student in the laboratory of Matthew Pratt, PhD. Her research focused on developing metabolic bioorthogonal chemical reporters to identify and to characterize post-translational modifications of proteins, including glycosylation, acetylation, and ubiquitination. For her postdoctoral studies, she worked in the laboratory of Ben Cravatt, PhD, at Scripps Research Institute in La Jolla, California. There she investigated the metabolism of covalent small molecules using activity-based protein profiling and identified the mechanisms of action of the multiple sclerosis drug Tecfidera® (dimethyl fumarate). She gained additional training with Irving Weissman, MD, at Stanford University School of Medicine in the fields of innate immunity and hematopoiesis before her arrival at UCSF in September 2019.
Hosted by Hannah Lembke
Professor Florence Williams
Tuesday, Oct. 10, 2023, 9:45 a.m. through Tuesday, Oct. 10, 2023, 9:45 a.m.
331 Smith Hall
Zoom Link
Professor Florence Williams
Department of Chemistry
University of Iowa
Abstract
Taking Advantage of Strong Boron-Oxygen and Boron-Fluorine Associations for Chemoselective Reactions
The Williams lab has been investigating the utility of highly Lewis acidic boron centers for the activation and cleavage of alkyl ethers and in halogen exchange reactions of trifluoromethylarenes. Such strategies have enabled the targeting of strong C–O bonds and C–F bonds for cleavage in the presence of weaker bonds. These practical methods have important applications in medicinal and agricultural chemistry, as well as in sustainable chemistry development, such as the mild separation of cellulose from lignocellulose – a biopolymer that can provide a renewable source of aromatic chemicals such as vanillin. This talk will examine the development of such boron-mediated methodologies, applications to different modern challenges, and the differential reactivity and behavior of boron trihalide species.
Florence Williams
Florence obtained her Ph. D. at University of California, Irvine working on organometallic catalysis with Prof. Elizabeth Jarvo. After post-doctoral research in chemical biology at Princeton in the lab of Prof. Dorothea Fiedler, Florence began her independent career at University of Alberta in Edmonton, Alberta, Canada and then in 2019 moved to University of Iowa. Her research involves using boron Lewis acids to selectively cleave strong σ bonds, including in complex materials settings, as well as mechanistic investigations of neurotrophic mall molecules that have potential applications in neurodegenerative disease.
Website: www.williamsresearchlab.com
Hosted by Professor Jessica Lamb
Professor Uttam K. Tambar
Thursday, Oct. 5, 2023, 9:45 a.m. through Thursday, Oct. 5, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Uttam K. Tambar
Department of Biochemistry
The University of Texas Southwestern Medical Center
Abstract
Stereoselective Reactions with Feedstock Chemicals
For several decades, chemists have designed new approaches to valuable materials that are economically efficient and environmentally benign. To this end, synthetic chemists have developed new synthetic strategies to access complex molecules from simple, inexpensive, and abundant feedstock chemicals. Our research group explores. new methods in this area. We present recent examples from our laboratory of stereoselective reactions with feedstock chemicals as starting materials. First, we discuss our approach to the stereoselective functionalization of unsaturated hydrocarbons through catalytic pericyclic reactions with chalcogen-based reagents. For example, we have developed enantioselective allylic functionalizations of terminal and internal alkenes. Second, we describe our approach to the enantioselective α-alkylation of aldehydes with amino acid derived alkylating reagents. We have devised a strategy for the activation of pyridinium salts derived from amino acids through the formation of light-activated charge transfer complexes with catalytically generated electron rich chiral enamines derived from aldehyde substrates and a chiral amine catalyst.
Uttam K. Tambar
Uttam Tambar moved from India to New York City in 1982. He received his A.B. degree from Harvard University in 2000, where he conducted research with Professors Cynthia Friend and Stuart Schreiber. He obtained his Ph.D. from the California Institute of Technology in 2006 under the guidance of Professor Brian Stoltz. After he completed his NIH Postdoctoral Fellowship at Columbia University with Professor James Leighton in 2009, Uttam began his independent research career at UT Southwestern Medical Center in Dallas. The Tambar lab is interested in catalysis, natural product synthesis, chemical biology, and medicinal chemistry. Uttam has received several awards, including the Thieme Chemistry Journal Award (2012), Sloan Foundation Research Fellowship (2013), Welch Foundation Norman Hackerman Award in Chemical Research (2019), Arthur C. Cope Scholar Award (2024), and Dallas Home Bartender Tiki Cocktail Prize (2019). Uttam is currently the Bonnie Bell Harding Professor in Biochemistry, Director of Diversity for Biochemistry, and Director of the Organic Chemistry Graduate Program, and Co-Leader of the Simmons Cancer Center’s Chemistry and Cancer Program.
Homepage: www.utsouthwestern.edu/labs/tambar/
Twitter/X: @TambarLab
Hosted by Professor Courtney Roberts
Professor Cathleen Crudden
Thursday, Sept. 28, 2023, 9:45 a.m. through Thursday, Sept. 28, 2023, 11:15 a.m.
331 Smith Hall
Zoom Link
Professor Cathleen Crudden
Queen’s University
Abstract
N-Heterocyclic Carbenes as Novel Ligands for Nanoparticles and Nanoclusters. Applications in Catalysis, Sensing and Molecular Medicine
NHCs have been documented as strong ligands for single metal atoms in molecular catalysts and as alternative to thiolates on planar metal surfaces. In this final talk, their use as ligands to stabilize atomically precise metal nanoclusters will be described. The structure of the cluster is shown to be strongly influenced by the nature of the NHC. By tuning the NHC structure (backbone and wingtip groups) a variety of new nanoclusters can be prepared. We will present the use of NHC-based nanoclusters with Au10, Au11, Au13, Au24 and Au25 cores, along with recent results on bimetallic cores. The unique properties of these NHC-stabilized clusters, including chirality, photophysical properties, stability and catalytic activity will be addressed.

Cathleen Crudden
Dr. Cathleen Crudden is the A.V. Douglas Distinguished Professor of Chemistry and Canada Research Chair (Tier 1) in metal organic chemistry at Queen’s University in Kingston, Ontario. She holds a Research Professorship at the Institute of Transformative Bio-Molecules (ITbM) in Nagoya, Japan, where she runs a satellite laboratory. She is a Fellow of the Royal Society of Canada, the Chemical Institute of Canada, the Royal Society of Chemistry (UK) and an elected member of the American Association for the Advancement of Science.
Dr. Crudden has made significant impact on diverse areas of science. She described one of the first cross-coupling reactions with chiral, enantiopure molecules that has made considerable impact on the preparation of pharmaceutical compounds. More recently, she has demonstrated the strength and versatility of N-heterocyclic carbene ligands in materials science, showing these ligands to be viable and versatile alternatives to thiolates as ligands for planar metal surfaces, nanoparticles and nanoclusters. This work has been called “game changing,” “elegant,” and “the new gold standard” by experts in the field.
Crudden has served as President of the Canadian Society for Chemistry and Chair of the Chemical Institute of Canada.She is Scientific Director of the Carbon to Metals Coatings Institute (C2MCI) at Queen’s University. She is currently Editor-in-Chief for ACS Catalysis. She has won numerous awards including the 2023 John Polanyi Award, a 2019 Cope Scholar award of the American Chemical Society, the Montreal Medal (2019), and the 2018 Carol Taylor award from the International Precious Metals Institute.
Hosted by Professor Gwen Bailey
Professor Cathleen Crudden
Wednesday, Sept. 27, 2023, 4 p.m. through Wednesday, Sept. 27, 2023, 5 p.m.
331 Smith Hall
Zoom Link
Professor Cathleen Crudden
Queen’s University
Abstract
N-Heterocyclic Carbenes as Novel Ligands on Planar Metal Surfaces–Self Assembled Monolayers, on Surface Ligand Dynamics and Applications in Atomic Layer Deposition
The use of N-heterocyclic carbenes to modify homogeneous metal catalysts is widespread since the high metal–NHC bond strength renders high oxidative and chemical stability to the resulting metal complexes. The use of NHCs to modify metal surfaces has received considerably less attention. We will describe the modification of planar metallic surfaces with NHCs. The nature of the surface overlayer is strongly dependent on the nature of the NHC. The ability of these NHC overlayers to act as small molecule inhibitors for atomic layer deposition processes will be described.
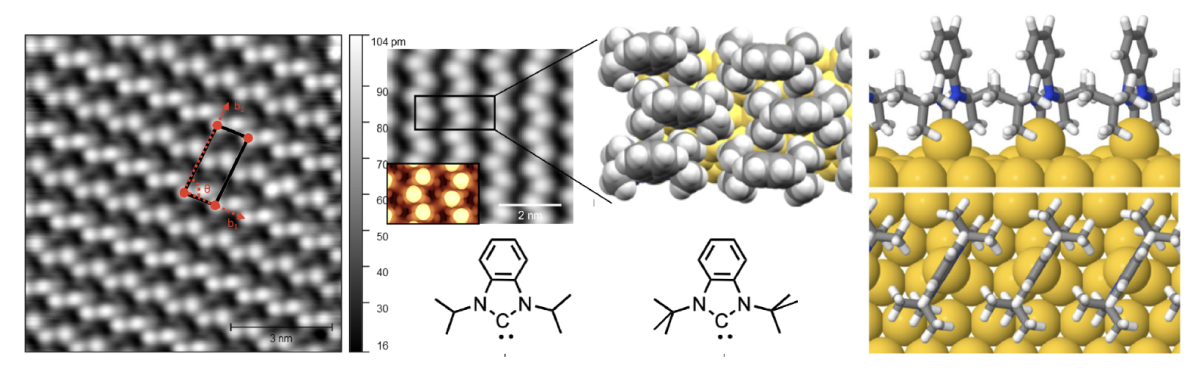
Cathleen Crudden
Dr. Cathleen Crudden is the A.V. Douglas Distinguished Professor of Chemistry and Canada Research Chair (Tier 1) in metal organic chemistry at Queen’s University in Kingston, Ontario. She holds a Research Professorship at the Institute of Transformative Bio-Molecules (ITbM) in Nagoya, Japan, where she runs a satellite laboratory. She is a Fellow of the Royal Society of Canada, the Chemical Institute of Canada, the Royal Society of Chemistry (UK) and an elected member of the American Association for the Advancement of Science.
Dr. Crudden has made significant impact on diverse areas of science. She described one of the first cross-coupling reactions with chiral, enantiopure molecules that has made considerable impact on the preparation of pharmaceutical compounds. More recently, she has demonstrated the strength and versatility of N-heterocyclic carbene ligands in materials science, showing these ligands to be viable and versatile alternatives to thiolates as ligands for planar metal surfaces, nanoparticles and nanoclusters. This work has been called “game changing,” “elegant,” and “the new gold standard” by experts in the field.
Crudden has served as President of the Canadian Society for Chemistry and Chair of the Chemical Institute of Canada.She is Scientific Director of the Carbon to Metals Coatings Institute (C2MCI) at Queen’s University. She is currently Editor-in-Chief for ACS Catalysis. She has won numerous awards including the 2023 John Polanyi Award, a 2019 Cope Scholar award of the American Chemical Society, the Montreal Medal (2019), and the 2018 Carol Taylor award from the International Precious Metals Institute.
Join us for a reception at the Coffman Union Campus Club after the seminar, from 5:00 – 7:00 p.m.
Hosted by Professor Gwen Bailey