Get to Know: The Santelli Environmental and Applied Biogeochemistry Lab
Microbes may be the smallest living organisms on Earth, but they exert a profound impact on the health of our planet - from producing oxygen in our atmosphere to ensuring healthy soils and clean drinking water. Life–supporting nutrients and contaminants alike are transformed by microbial processes, which influences their bioavailability, quantity, distribution, and toxicity in aquatic and terrestrial ecosystems. These biotic processes are complex and often coupled with abiotic processes, leading to mineral formation, mineral dissolution, and chemical speciation and phase changes. Identifying the underlying transformation mechanisms and understanding their impact on environmental geochemistry is challenging, but this knowledge is necessary for sustaining and improving environmental and societal health.

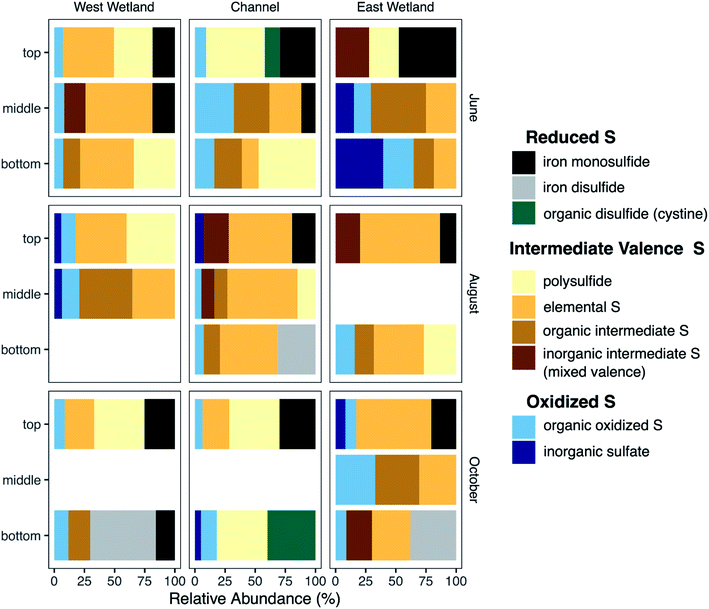
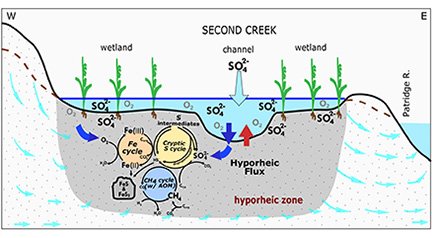
Research in Associate Professor Cara Santelli’s Environmental and Applied Biogeochemistry lab uses laboratory and observational field-based approaches to shed light on these coupled microbial and abiotic processes that transform nutrients and contaminants. We examine these processes in a wide range of environments, from freshwater wetlands, lakes, and streams to urban farm soils to the deep continental lithosphere. In one recently published study, we demonstrated that microbially-driven sulfur cycling (sulfate reduction and reoxidation that can regenerate the sulfate) occurs in sulfate-impacted riparian wetland and stream sediments in northern Minnesota’s “Iron Range”. For example, we see that the subsurface sediments are not dominated by reduced sulfur compounds such as iron monosulfide (e.g., mackinawite) or iron disulfide (e.g., pyrite), but rather intermediate valence sulfur forms such as elemental sulfur (S(0)) that form due to anaerobic sulfide oxidation. Further, dissolved sulfate is always present (i.e., regenerated) in the interstitial porewaters despite evidence of active microbially-driven sulfate reduction that should fully deplete sulfate from the environment. Sulfur cycling has often been overlooked in freshwater environments due to low sulfate levels in water, but our study revealed this “cryptic” process is robust and has substantial implications for carbon burial and turnover, methane emissions, and overall water quality from these environments. This study was done in collaboration with Dr. Crystal Ng (Associate Professor of Hydrogeology and co-PI), former MS student Josh Torgeson, former research associate Dr. Carla Rosenfeld, and several Earth and Environmental Sciences graduate and undergraduate students.
Currently funded by the Department of Energy, we are expanding this work to study unpolluted (i.e. very low sulfate) waters to determine how these hydrobiogeochemical processes more broadly impact nutrient and contaminant fate and distribution and better model these hydrobiogeochemical processes for assessing and predicting their impact as we experience global environmental change. We are also using knowledge from these studies to help inform our collaborative research with tribes, intertribal treaty organizations, and numerous University of Minnesota faculty, staff, and students to protect Manoomin / Psiη (Wild Rice).