Experiment follows theory

CSE researchers hunt for better touchscreen materials lead to novel possibilities for portable disinfecting light sources
October 8, 2021
Theorists don’t usually get the credit when a new invention turns out to be a hit. Usually, it is the experimentalists who present the results to the public when the outcome of a research endeavor is successful—and, often, the theorists’ work gets overlooked among all the excitement of a new discovery. But, this time, during the COVID-19 pandemic, it was two theorists at the University of Minnesota College of Science and Engineering who got the spotlight.
Turan Birol, an assistant professor of chemical engineering and materials science, and Arpita Paul, a postdoctoral researcher in Birol's lab, introduced a new material that can be used as the building block of a portable solution to disinfect and sanitize areas from bacteria and viruses such as COVID-19.
“There is a very big push to find new, transparent conductors—new materials that are transparent to light but at the same time, they are electrically conducting,” Birol explained.
He said the reason for that is twofold. Materials such as indium tin oxide that work really well for touch screens are very expensive and indium is a resource about to be depleted. Also, he noted that elements like copper are commonly used as conductors because they are highly conductive—but they are shiny and so lack transparency.
By applying novel ideas of materials currently used for the coating on touch screens, Birol approached this engineering problem by turning it into a physics problem. Birol and Paul performed quantum mechanical calculations based on their current knowledge of those materials and entered those equations into the supercomputers at the Minnesota Supercomputing Institute on the Twin Cities campus.
From the test results and his knowledge of advanced physics, Birol evaluated the material strontium vanadate (SrVO3) as a potential candidate for a coveted touch screen and tested its performance. He discovered that it had an absorption rate that was not entirely transparent on the visible energy spectrum, meaning it was still visible to the naked eye. So he continued looking.
Soon he discovered strontium niobate (SrNbO3). This compound showed no sign of absorbing visible light in his models. It reflected only red and infrared light, and it was transparent in ultraviolet, or UV, range on the energy spectrum.
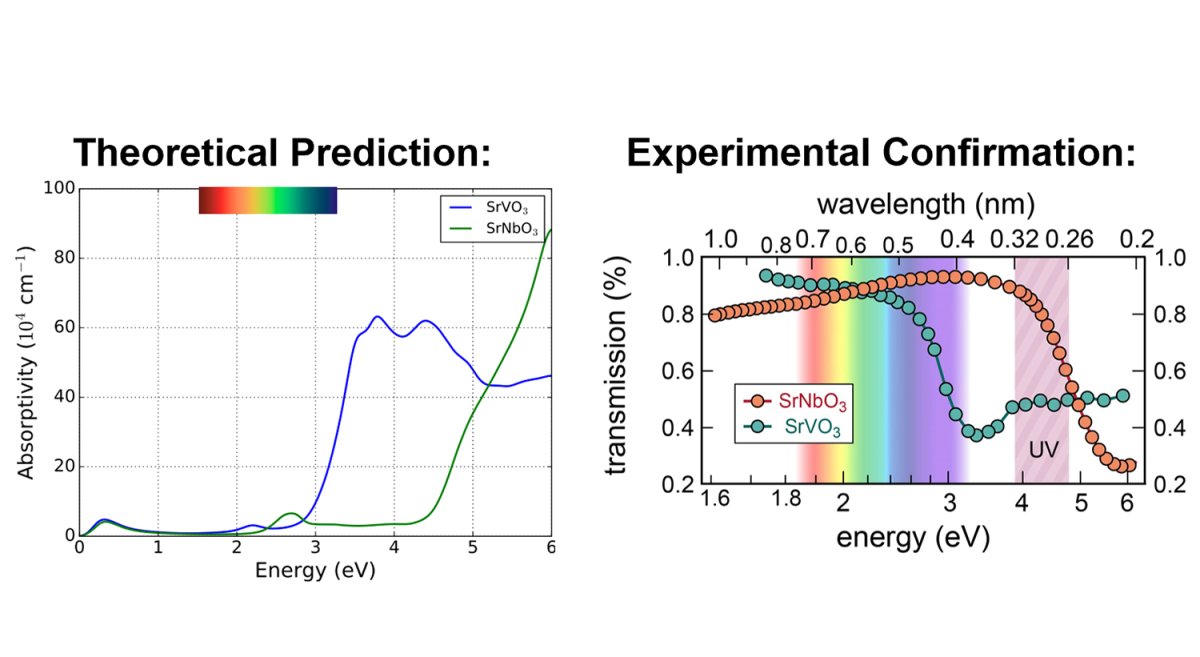
Right: The chart, which confirms Birol’s prediction, shows the results of comparing strontium niobate with another strontium vanadate. Credit: Park, Gopalan, Birol, Engel-Herbert, et al., Comm. Phys.
Birol got in touch with his colleagues at Penn State who confirmed his theoretical prediction experimentally. They included Joseph Roth and Roman Engel-Herbert, with whom he had an ongoing collaboration in the search for transparent conductors.
His finding was groundbreaking because prior to Birol’s discovery, the only UV light sources that were available were not very convenient. They often required high power or energy sources, did not last very long, and included mercury, an expensive and potentially harmful substance.
“For many of these LED applications, you need a transparent conductor as the electrodes,” he said.
“Strontium niobate is pretty much perfectly transparent in the ultraviolet region and for an ultraviolet light source, an ultraviolet transparent conductor would be extremely useful,” Birol explained.
As a handheld device, a LED containing strontium niobate could be easily transported and used in large venues and frequently populated areas such as public transportation systems, hotels, sports arenas, and theatres. It could also be used in HVAC systems in buildings and used to disinfect and sanitize the air.
The timing of his finding was serendipitous. It came as the novel coronavirus was first becoming known to the public and increasingly growing into a global health concern. Businesses were scrambling to retain customers. They were looking for another way to keep their customers safe—and UV radiation light sources offered them that.
The shared effort of Birol and his collaborators resulted in a $100,000 extension on their National Science Foundation “Designing Materials to Revolutionize and Engineer our Future” grant.
It’s not every day that theory leads to a successful outcome. Scientists can spend years developing an idea and the basic science ends up going nowhere. In this case, however, it did.
“We had the problem statements. We had the theoretical prediction, and then the experiment followed up,” Birol said. “So, it was theory guiding experiment, which makes a theorist happy.”
Birol’s work was published in Physical Review Materials, a journal by the American Physical Society.
Story by Autumn Brower
If you’d like to support research in the University of Minnesota College of Science and Engineering, visit our CSE Giving website.
