Recycling mixed plastics is about to get a lot easier

Photo credit: GraphicStock
CSE-invented polymers will make recycling processes more efficient
June 25, 2021
The United States produced an average of 35.7 million tons of plastic in 2018. According to the Environmental Protection Agency, only about 9 percent of those plastics were actually recycled. University of Minnesota College of Science and Engineering researchers recently developed a technology that aims to increase that percentage.
Not all plastics are created equal. There are seven main types—these correspond with the numbers inside recycling logos on most plastic products—and they all need to be recycled separately. Because of this, recycling facilities spend a lot of time sorting plastics before they can be repurposed.
However, a research team led by Professor Chris Ellison in CSE’s Department of Chemical Engineering and Materials Science recently came up with a way to recycle two of those plastic types—polyethylene terephthalate (PET) and polyethylene (PE)—together.
“Polyethylene and polyethylene terephthalate represent two of the largest manufactured polymers in the world by volume,” explained Ellison. “They’re the two major classes of materials you find a lot in food packaging, and consumers are using them multiple times every day.”
“The question becomes, ‘What can we do to address this mixed-waste problem that is intrinsically there due to the materials that we use every day?’” he said.
Ellison, whose lab is a part of the University’s Center for Sustainable Polymers on the Twin Cities campus, holds the Rumy Innovation Chair in CEMS and the Piercy Professorship in Chemical Engineering, both endowed by CSE donors.
Like a good vinaigrette
Plastics are made up of long chains of polymers, which are essentially large molecules bonded together. Generally, polymers of different types don’t like to mix with each other. To address this issue, Ellison’s team developed what is called a multiblock copolymer, an additive that can bond the two types of plastic together.
The copolymer is similar to a surfactant that helps combine two substances—like the Dijon mustard that helps blend oil and water in a salad vinaigrette. It can be added in small amounts either during the recycling process or even when the materials are being initially manufactured.
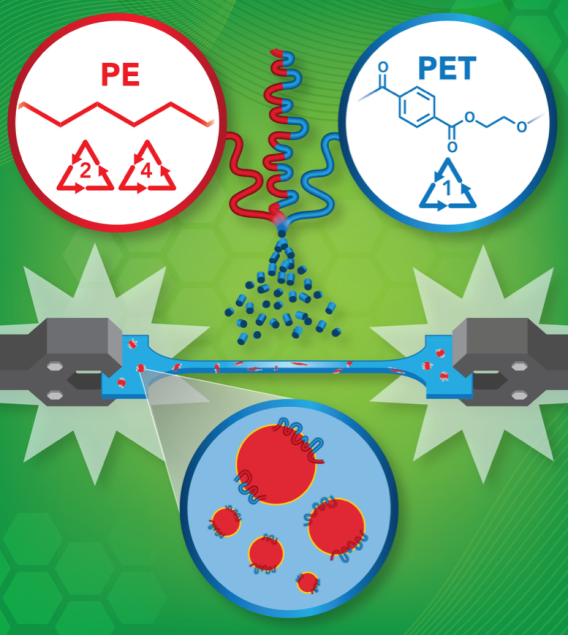
In some cases, multiple plastic types are used together in one product. Food packaging such as granola bar or candy wrappers are common culprits of this, using layers of both PET and PE. Normally, these wouldn’t be recyclable. But with Ellison’s technology, they can be.
“In an ideal world, you could just reimagine the plastics industry right from the beginning,” Ellison said. “But the reality is that these kinds of materials have been engineered for over 60 years.”
“That’s why we like this idea of recycling with these new additives,” he said. “It doesn't require us to reimagine the entire industry, but rather develop a new capability for the industry as it exists today.”
Unsurprisingly, the researchers’ work has caught the attention of that industry. With help from the University’s Technology Commercialization office, the copolymer technology was picked up by a local company, who’s working on putting it to use in the field. Plus, one of the study’s lead authors was a fellow from the Industrial Partnership for Research in Interfacial and Materials Engineering (IPRIME), a CSE program that allows scientists from industry to work on a project with researchers at the University.
But according to Ellison, industry interest and technological innovation is just the start in a long journey to a more sustainable future.
“You're seeing a lot of demand from consumers to reduce waste and address sustainability of products that they buy, and you're starting to see policy in different parts of the world pushing in that direction,” Ellison said. “It’s a very complicated problem, and it doesn't only require technological innovation. But I think the fact that a company has licensed this and is interested in commercialization is a really good sign.”
Story by Olivia Hultgren
If you’d like to support research in the University of Minnesota College of Science and Engineering, visit our CSE Giving website.
