UMN awarded $3.7M to prepare for clinical trials of lab-created pediatric heart vessels that grow with the recipients
Research could prevent need for repeated surgeries in children with heart defects
MINNEAPOLIS/ST. PAUL (07/18/2022) — A University of Minnesota Twin Cities-led team of researchers has received a $3.7 million grant over the next four years from the U.S. Department of Defense to prepare for human clinical trials of artificial blood vessels bioengineered in the lab that grow with the patient. If successful, these new vessel grafts would prevent the need for repeated surgeries in children with congenital heart defects.
The funding is part of the Department of Defense’s Congressionally Directed Medical Research Programs that fund programs that are “transforming healthcare through innovative and impactful research.”
One of the greatest challenges in vessel bioengineering is designing a vessel that will grow with its new owner. Recipients who have heart defects at birth often outgrow current vessel grafts and need to have larger vessels implanted several times as they grow.
“This grant is a major step forward and will allow us to do everything that's necessary to get to Day 1 of a first clinical trial where we would implant one of our lab-created blood vessels into an infant with a heart defect called discontinuous pulmonary arteries or hemitruncus” said Robert Tranquillo, a Distinguished McKnight University Professor in the Department of Biomedical Engineering and the Department of Chemical Engineering and Materials Science.
“If all goes well, this clinical trial could begin within about 18 months,” Tranquillo said.
Children with discontinuous pulmonary arteries or hemitruncus are missing a branch of the pulmonary artery to the left or right lung at birth. According to the STS National Database, at least 55 babies are born each year with one of these two heart defects.
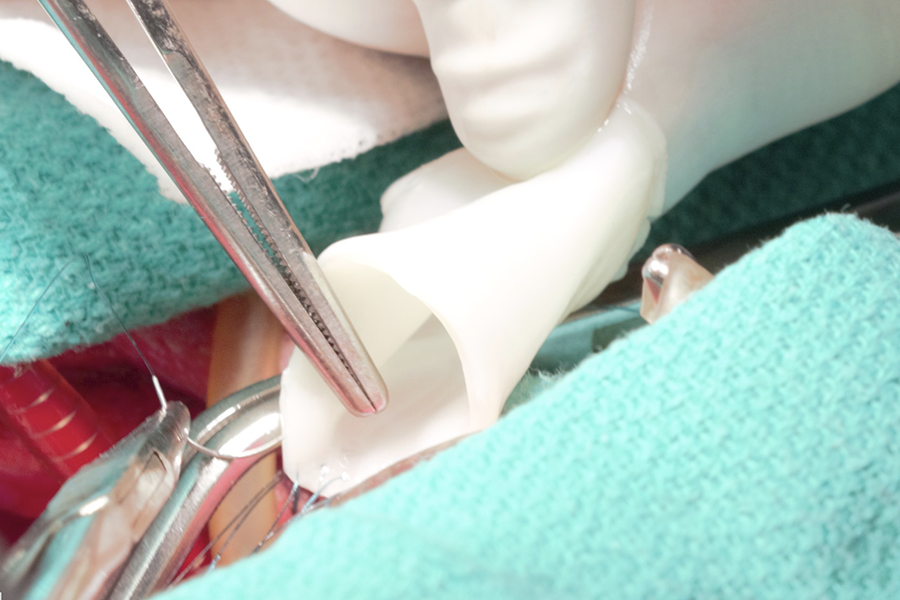
To prepare for the clinical trials, the vessel-like tubes will be manufactured by the University of Minnesota start-up company, Vascudyne, Inc. (St. Paul MN) that licensed technology based on ground-breaking research from Tranquillo’s team. The tubes are grown in the lab from a donor’s skin cells and then they remove the cells to minimize the chance of rejection. The tubes can be stored and implanted when they are needed. When implanted, the tube is repopulated by the recipient’s own cells allowing it to grow.
To develop the material for the vessel-like tubes, researchers combined cells in a gelatin-like material, called fibrin, in the form of a tube and then provide nutrients necessary for cell growth using a bioreactor. Zeeshan Syedain, a senior research associate in Tranquillo’s lab who now also serves as Vascudyne’s chief scientific officer, co-developed the bioartificial vessel technology. Syedain is now part of the research team on this new grant.
In addition to developing the tubes, the grant will cover any remaining pre-clinical studies needed before the human clinical trials. Tranquillo and Syedain will partner with Experimental Surgical Services in the University of Minnesota Medical School to test the tubes in lambs by replicating a similar study in 2016 when lamb’s cells repopulated tubes implanted into the main pulmonary artery and proved that the tubes grew with the lamb.
This study is already underway with jumpstart funding from the Frank J. & Eleanor A. Maslowski Charitable Trust, with two lambs implanted with a tube into the left pulmonary artery branch that has grown with the lambs into adulthood, now beyond one year. They will also partner with the University’s Preclinical Research Center to test the intended anti-coagulation therapy to be used in the clinical trial.
In addition, the research team is partnering with pediatric cardiac surgeons at Children’s Minnesota and Boston Children’s Hospital to help understand the pre-clinical data and help design the first human clinical trial.
“This research could have a major impact for children with heart defects. Instead of three, four or even five surgeries or interventions during their childhood, children would only need one surgery,” Tranquillo said. “This would substantially reduce trauma and risk for children and the overwhelming health care costs for families.”
If the clinical trials with the vessels are successful in the future, Tranquillo said he hopes to move toward clinical trials of similar research developed in his lab involving bioartificial pediatric heart valves constructed from these tubes that grow with the recipient. Implanting heart valves that grow with the child could help thousands of children each year who are born with heart valve defects, such as Tetralogy of Fallot.
In addition to Tranquillo and Syedain, the research team includes Richard Bianco, associate professor of surgery and program director of Experimental Surgical Services in the University of Minnesota Medical School; Melanie Graham, associate professor of surgery and program director of the Preclinical Research Center in the University of Minnesota Medical School; John Mayer, senior associate in the Department of Cardiac Surgery at Boston Children’s Hospital and professor of surgery at Harvard Medical School; Robroy MacIver, pediatric cardiac surgeon at Children’s Minnesota; Richard Murphy, chief operating officer of Vascudyne; and Robert Schumacher, scientific director of the Center for Translational Medicine at the University of Minnesota.
