FACULTY: The Science of Small

From bank employee to engineering nanoparticles
Chemistry professor and associate department head Christy Haynes designs tiny polymer beads that can save lives and feed the hungry—and Fortune 500 partners are paying attention.
“You can’t see any of the nanoparticles we make,” she said, “but they can be used to deliver drugs to fight disease or transport nutrients to increase crop yield. They can also be used to sense things or to make things visible. For example, we have a collaboration with Ecolab on some nanoparticles we make, and the goal is to incorporate them into products so you can trace where the products have been used.”
Haynes’ team has also brought their nanoscience expertise into a collaboration with 3M, where she designed nanostructures for the detection of toxins in food. The sensors she and her students developed make it possible to detect multiple toxins within a complicated food sample with high sensitivity.
Haynes’ life today is a far cry from the future her mom and dad saw for her.
“My parents were really young when they had me, and neither of them had the opportunity to go to college,” said Haynes, who grew up in Arizona’s Sonoran Desert. “So, going to college definitely wasn’t part of what was expected of me or even what I expected of myself. My parents were actually against me going to college. I was a bank teller in high school and they kept telling me, ‘You’re really good at this; you could be a bank manager someday.’”
Haynes believed this was her path, until she didn’t.
“One day it just became really clear to me that education was the most likely exit ramp from the life my parents led,” she said, “which included jobs that didn’t seem very fulfilling and neighborhoods that didn’t feel particularly welcoming.”
In 2005, with advanced degrees plus national awards, Haynes brought her expertise in nanomaterials to Minnesota. The field of nanoscience was growing. Nanoparticles—matter that’s a few billionths of a meter in size—were in all sorts of everyday products, including handwashes, sunscreen, scratchproof eyeglasses, and wrinkle-free fabrics. Haynes began wondering: What if nanoparticles got inside humans? What are the health effects? What are the potential risks to our environment?
In hopes of finding those answers, Haynes helped established the National Science Foundation Center for Sustainable Nanotechnology in 2012. Seven years later, she spent a year at the Universitat Politécnica de Valéncia, Spain studying the effects of nanotechnology on our living world.
“There are cases where nanoparticles can do bad things but, for the most part, nobody’s discovered new modes of human toxicology,” she noted. “But the truth is things build up in the environment—concentrations can get high in very specific pockets of the ecosystem—and the question is, ‘Okay, what happens then?” The good news is we could actually regulate nanoparticles that we’re putting into a person the same way we could regulate anything else for human use.”
In addition to championing more ecological practices in her field, Haynes, a Distinguished McKnight University Professor, excels at being an advocate for diversity in higher education. She regularly partakes in outreach activities in local schools and is a lead presenter for the University of Minnesota’s Energy and U show, which draws more than 10,000 third- to sixth-graders to the Twin Cities campus.
“I had a lot of luck when I was teenager,” she said. “I didn’t have any of the kind of support system that told me what to do or how to apply for college, or what to study, but I had a certain kind of academic ability. My life turned out way better than I expected—and I have a platform now to make a difference. Education gives you more options for what you can do with your life.”
From soccer balls to atomic-scale structures
There are special rooms for the instruments that K. Andre Mkhoyan uses. The walls are padded to dampen acoustic vibrations, the air is cooled so temperatures never vary more than one to two degrees, and the floors are concrete to lessen any sudden human movements or unexpected seismic activity.
Mkhoyan, the Ray D. and Mary T. Johnson/Mayon Plastics Chair in CSE’s Department of Chemical Engineering and Materials Science, specializes in advanced transmission electron microscopy (TEM). Instead of using visible light, his microscopes are capable of producing high-resolution images of a specimen with a beam of electrons—and at magnifications of up to 10 million times what the unassisted eye can see.
“To see human hair very nicely, you will need about 1,000 times magnification because human hair is about 75 microns or 75,000 nanometers,” he explained. “One nanometer is a billionth of a meter or one millionth of a millimeter. In my research, we want one level higher. We want to go to the atomic scale, which is 10 times higher than nanometer.”
In other words, Mkhoyan can really see the flaws that could compromise the integrity of a structure. Finding these discrepancies, or defects, in commonly used materials is one direction that really excites him today. Why? Because what makes one material more desirable versus another depends on its properties.
“Let’s say you have a piece of metal and it has a crack or some holes on it,” he explained. “Now, imagine those defects inside the metal. Every material has plenty of them on an atomic level. What this means is some atoms are missing or they may be in the wrong position. Knowing where those defects are allows us to better understand their role or sometimes take advantage of them. We can use a defect as a way to improve the material.”
Recently, his research team was the first to observe metallic lines in a crystal. The discovery is significant because it could lead to even more smart devices and windows, ones that have a touch-sensitive surface, are transparent to light and conduct electricity.
“We need to understand what a material looks like first to help drive technology,” said the Armenian-born Mkhoyan, who joined the University of Minnesota in 2008. “I always like to look at defects as spices in the food. If you sprinkle the right amount of the defects into a new structure, like spices into your soup or meal, it will give you all of the flavor you need or the functional properties you want.”
Arriving at his current career wasn’t easy. Mkhoyan spent a whole summer in his early teens “meditating on” whether to pursue his dreams of becoming a soccer player or a scientist.
“I needed to choose one or the other because there was a really good boarding school for physics and math on the other end of town,” he recalled, “and it was absolutely incompatible with soccer.”
His decision eventually led to graduate school in the United States and his first full-time job as a physicist at Bell Labs, in Murray Hill, N.J., where he was introduced to TEM.
“Nanotechnology was starting to be a really important topic and I really enjoyed that type of science,” he said. “I also always had a fascination with photography. So it felt like a good idea then to combine both my joys in life and make a career out of it.”
Mkhoyan—who hasn’t missed a World Cup soccer final in 30 years—oversaw the 2014 renovation of his lab in the University of Minnesota’s Characterization Facility. His role included securing funding and designing the rooms to hold key instruments, which are available to anyone whose work spans from nanotechnology to medicine.
However, advanced microscopes are complicated instruments. Mkhoyan said some take one month to learn; others, half a year or a year to use properly.
“I think it’s fair to say Minnesota is one of the most well-equipped in electron microscopes,” he said. “There are not that many universities that can say that. We are definitely on the forefront of this type of research and facility.”
From parachutes to bacteria
Jake Bailey studies bacteria. In fact, the associate professor of earth and environmental sciences is intrigued by the largest known bacterium—Thiomargarita namibiensis—and relishes jumping into a research vessel every year to collect it off the coast of Namibia, in southwestern Africa.
“These are the only bacteria that you can see with the naked eye,” he said. “They are very small, like a little speck of dust or about a millimeter in diameter, but you can see them.”
Last fall, Bailey’s team discovered that other bacteria closely related to Thiomargarita dissolved rocks, releasing excess carbon into the ocean and atmosphere. This finding enables scientists to better estimate the amount of carbon dioxide in Earth’s atmosphere, a main driver of global warming.
“These bacteria are fascinating for many reasons, but one reason is that we believe they make a mineral form of phosphorus on the ocean floor,” he explained. “Right now, we get most of our phosphorus—for agriculture that feeds seven-and-a-half billion people—from land deposits in a variety of different places, like Morocco and China, and we’re basically mining our way through these non-renewable deposits.”
A seafloor mining option is being considered to stretch those resources, he added, but like any other kind of excavating or extracting of natural resources, tapping ocean phosphorus is not without controversy because of its understudied environmental impacts.
However, Bailey’s life work isn’t about tackling disputes. Instead, this Colorado-born geobiologist is focused on basic scientific discoveries that can lead to bigger solutions.
“Microbes are living everywhere on our planet, and they have for the last three-and-a-half billion years,” said Bailey, who fell in love with science when reading books by Stephen Hawking and Richard Feynman during his stint as a paratrooper for the U.S. Army’s 82nd Airborne Division. “They live all the way in the upper atmosphere and way into the earth as well. There are millions and millions of bacteria in just a teaspoon of soil or in sea water, and we’re still just on the fringes of understanding all the different things that microbes are doing on our planet.”
Humans have used microorganisms to produce food, treat waste water, and clean up polluted sites. More recently, noted Bailey, scientists have found that microbes can manipulate the chemical makeup of minerals and cause them either to precipitate or dissolve.
Those discoveries got Bailey thinking: Would the same kind of chemistry that occurred with bacteria on the ocean floor also happen in our mouths?
“My research group looked into this, and sure enough, we found a lot of polyphosphate-accumulating bacteria in our dental plaque and saliva. This really hadn’t been looked at before—how these bacteria might change the chemical environment of our teeth and influence either their dissolution, like making cavities, or also the precipitation under different conditions, making mineral deposits like dental calculus or tartar.”
The study, funded by the National Institutes of Health and the University of Minnesota Office of the Vice President for Research, was significant because up until then dental decay was only thought to be caused by acids from bacteria that broke down sugars on our teeth. Bailey’s team, working with Dr. Rob Jones in the U of M School of Dentistry, suggested that oral bacteria also played a different role—by removing phosphate from the mouth and altering the chemistry of saliva.
Although preventable, tooth decay remains one of the most common chronic disease of children ages 6 to 11 and adolescents 12 to 19 years, according to the U.S. Centers for Disease Control and Prevention.
“Microbes are so much more than pathogens, and there are so many different types of microbes out there that are doing amazing things—many of which have nothing to do with human health,” Bailey noted. “They’re just trying to make a living out there, just like all of us. If we can figure out their secrets, we can have some powerful tools that we can apply to many of the challenges we’re facing in our society, like climate change.”
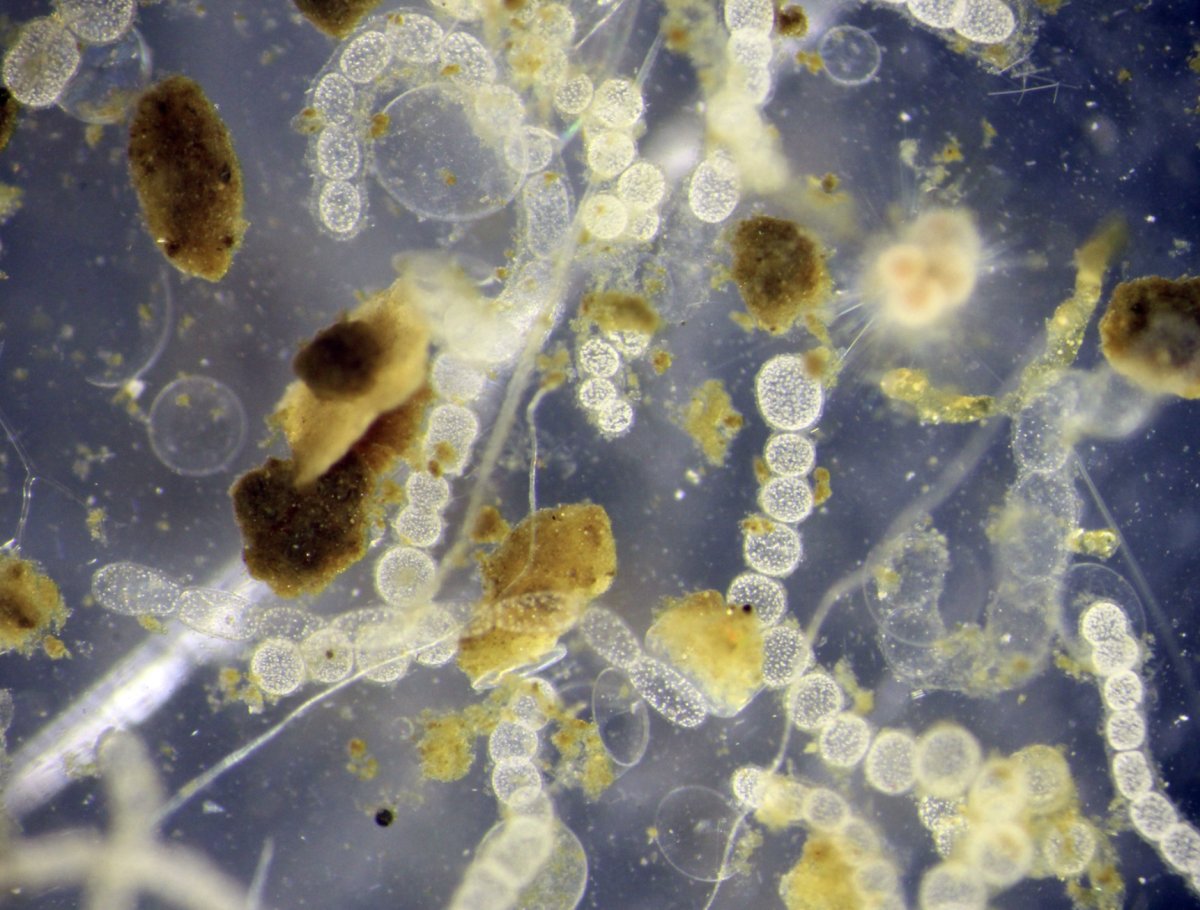
Jake Bailey is collaborating with University of Minnesota research scientist Beverly Flood to sequence the genome of his favorite tiny organism, shown above.



