U of M researchers develop technique for rapid detection of neurodegenerative diseases like Parkinson’s and Chronic Wasting Disease
Researchers’ groundbreaking new diagnostic technique will allow for faster and more accurate disease detection
MINNEAPOLIS / ST. PAUL (05/01/2023)—University of Minnesota Twin Cities researchers have developed a groundbreaking new technique that will allow for faster and more accurate detection of neurodegenerative diseases. The method will likely open a door for earlier diagnosis and mitigation of various diseases that affect humans, such as Alzheimer's and Parkinson's, and similar diseases that affect animals, such as chronic wasting disease (CWD).
Their new study is published in Nano Letters, a premier journal in the field of nanotechnology published by the American Chemical Society.
“This paper mainly focuses on chronic wasting disease in deer, but ultimately our goal is to expand the technology for a broad spectrum of neurodegenerative diseases, Alzheimer’s and Parkinson’s being the two main targets,” said Sang-Hyun Oh, senior co-author of the paper and a Distinguished McKnight University Professor in the University of Minnesota Department of Electrical and Computer Engineering. “Our vision is to develop ultra-sensitive, powerful diagnostic techniques for a variety of neurodegenerative diseases so that we can detect biomarkers early on, perhaps allowing more time for the deployment of therapeutic agents that can slow down the disease progression. We want to help improve the lives of millions of people affected by neurodegenerative diseases.”
Neurodegenerative diseases such as Alzheimer's, Parkinson's, mad cow disease, and CWD (widely found in deer) share a common feature—the buildup of misfolded proteins in the central nervous system. Detecting these misfolded proteins is crucial for understanding and diagnosing these devastating disorders. However, existing diagnostic methods, like enzyme-linked immunosorbent assay and immunohistochemistry, can be expensive, time-consuming, and limiting in terms of antibody specificity.
The University of Minnesota researchers’ method, dubbed Nano-QuIC (Nanoparticle-enhanced Quaking-Induced Conversion), significantly improves the performance of advanced protein-misfolding detection methods, such as the NIH Rocky Mountain Laboratories’ Real-Time Quaking-Induced Conversion (RT-QuIC) assay.
The RT-QuIC method involves shaking a mixture of normal proteins with a small amount of misfolded protein, triggering a chain reaction that causes the proteins to multiply and allowing for the detection of these irregular proteins. Using tissue samples from deer, the University of Minnesota team demonstrated that adding 50-nanometer silica nanoparticles to RT-QuIC experiments dramatically reduces detection times from about 14 hours to only four hours and increases the sensitivity by a factor of 10.
A typical 14-hour detection cycle means that a lab technician can run only one test per normal working day. However, with a detection time of less than four hours, researchers can now run three or even four tests per day.
Having a quicker and highly accurate detection method is particularly important for understanding and controlling transmission of CWD, a disease that is spreading in deer across North America, Scandinavia, and South Korea. The researchers believe that Nano-QuIC could eventually prove useful for detecting protein-misfolding diseases in humans, specifically Parkinson's, Creutzfeldt-Jakob Disease, Alzheimer's, and ALS.
“Testing for these neurodegenerative diseases in both animals and humans has been a major challenge to our society,” said Peter Larsen, senior co-author of the paper and an assistant professor in the University of Minnesota Department of Veterinary and Biomedical Sciences. “What we’re seeing now is this really exciting time when new, next generation diagnostic tests are emerging for these diseases. The impact that our research has is that it’s greatly improving upon those next generation tests, it’s making them more sensitive, and it’s making them more accessible.”
The research was funded by the Minnesota Environment and Natural Resources Trust Fund as recommended by the Legislative-Citizen Commission on Minnesota Resources (LCCMR); the Minnesota Agricultural Experiment Station Rapid Agricultural Response Fund; and the Minnesota Agricultural, Research, Education, Extension and Technology Transfer (AGREETT) program.
“Minnesotans value science and support basic and applied research. As legislators, we have invested Environmental Trust Fund dollars to provide solutions for complex problems like chronic wasting disease,” said Representative Rick Hansen, chair of the Minnesota House Environment and Natural Resources Committee and co-chair of the LCCMR. “I am proud of the work of the LCCMR and the legislature in supporting this research and will continue to advocate for funding to research and prevent future problems affecting our wildlife and ourselves.”
Larsen and Oh lead the University’s Minnesota Center for Prion Research and Outreach (MNPRO) molecular diagnostic research and development team, which leverages this government funding to conduct research on protein misfolding diseases that greatly impact the state of Minnesota.
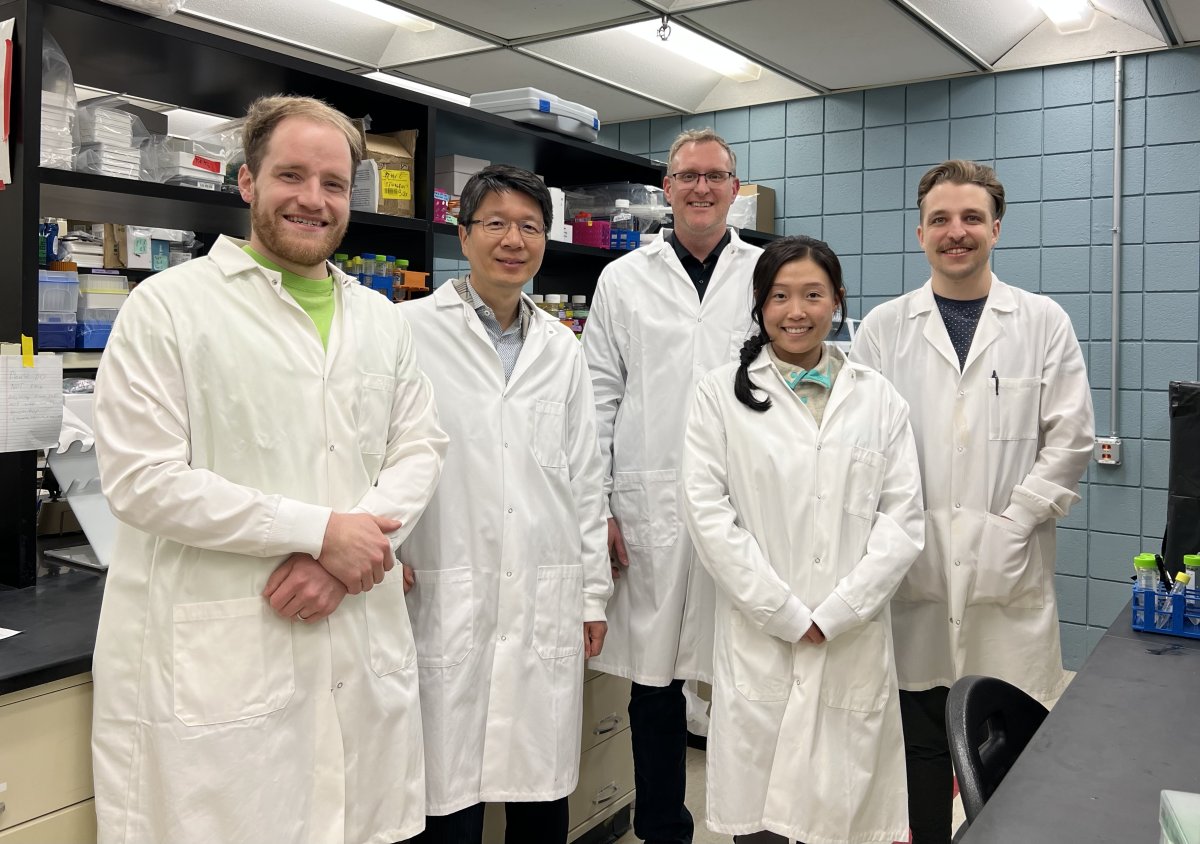
In addition to Oh and Larsen, the team involved in this paper included University of Minnesota Twin Cities researchers Peter Christenson (lead author and Ph.D. candidate in the Department of Electrical and Computer Engineering), Manci Li (Ph.D. candidate in the Comparative and Molecular Biosciences Program), and Gage Rowden (researcher in the Department of Veterinary and Biomedical Sciences).
Read the full paper entitled, “Nanoparticle-enhanced RT-QuIC Diagnostic Assay for Misfolded Proteins,” on the Nano Letters website.
Learn more about the Minnesota Center for Prion Research and Outreach.
