Exploring the Role of Cognition and Sensory Feedback on Upper Limb Motor Control

Assistant Professor
Department of Rehabilitation Medicine
More than 75% of stroke survivors experience upper limb motor deficits, ranging from arm weakness to the loss of dexterity, that impair their ability to conduct activities of daily living (Lang et al., 2013; Lawrence et al., 2001). Movement rehabilitation post stroke is generally focused on remediating deficits related to motor execution in the paretic arm. However, functional movement requires intact cognition, which means that cognitive deficits post stroke can hinder the ability to regain functional independence (Barker-Collo & Feigin, 2006). A large body of research has provided evidence for the laterality of motor execution processes (Sainburg, 2002; Bagesteiro & Sainburg, 2003), but we do not completely understand how the laterality of cognitive processes affects motor function. This is an important focus area because accurate, well-coordinated movement requires a complex interaction between cognitive, sensory, and motor execution processes.
Cognitive processes, such as action selection, have been found to be lateralized to right frontal regions (Philipose et al., 2007), and damage to the right hemisphere produces substantial deficits in responding to unexpected changes in motor goals, updating motor strategies, and movement preparation (Schaefer et al., 2012). Damage to the brain, due to stroke, results in limited neural resources that need to be efficiently allocated when producing a movement; therefore, an increased cognitive load can affect movement performance differently depending on the side of brain damage. By examining lateralized cognitive processes and understanding how they affect functional outcomes in left and right hemisphere damaged stroke survivors, we can design more personalized tools and strategies for movement rehabilitation.
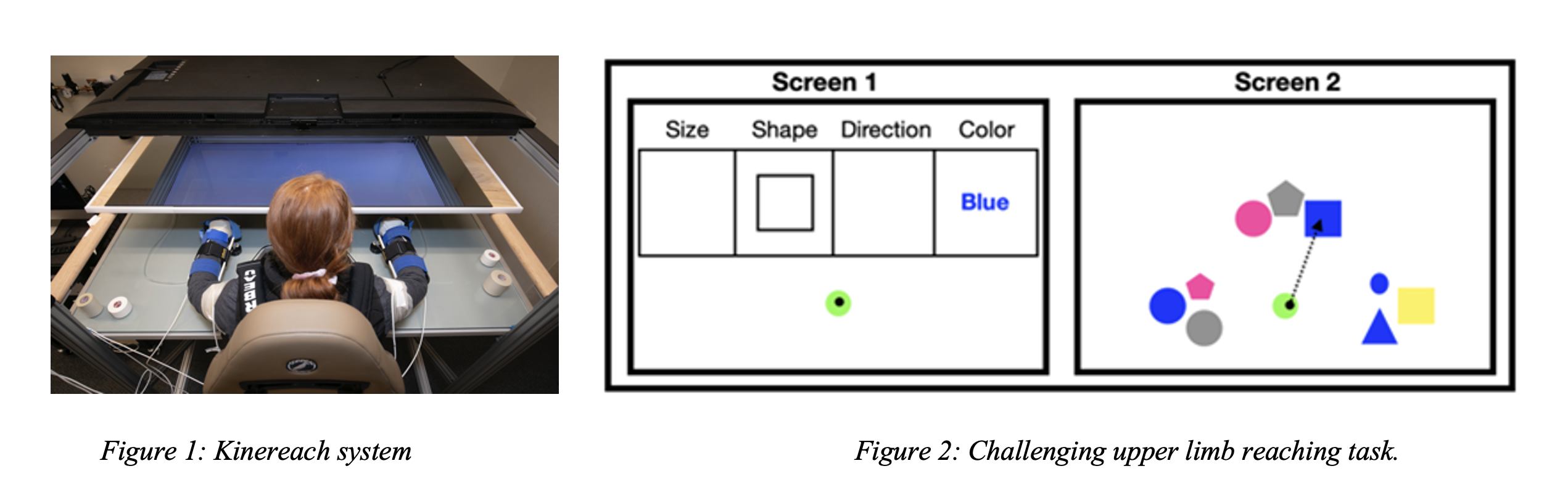
Ongoing research in our lab is focused on examining the role of cognitive and motor execution processes in producing functional movement in chronic stroke survivors with severe hemiparesis. We use a 10 degree-of-freedom virtual reality motion capture system (Kinereach, Figure 1) to obtain upper limb kinematics data, and wireless surface electromyography sensors to record electrical activity of elbow and shoulder muscles. We are also integrating an eye tracking device (Tobii Pro Glasses) to our motion capture system in order to obtain changes in pupil size during cognitively challenging motor tasks (see Figure 2). Our current setup enables us to examine neural control during visual perturbations, increased task complexity, anticipatory movement planning, and feedback-based movement control.
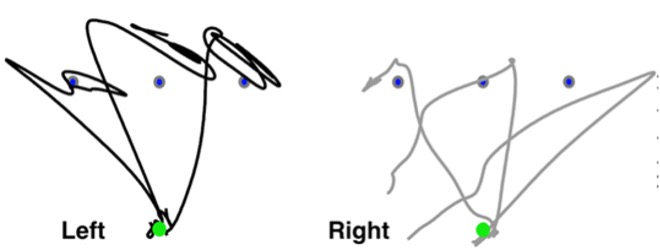
My graduate work focused on examining the role of sensory feedback in upper limb motor control and motor learning in children with dystonia. We used a tactile feedback device that obtains surface EMG activity from a muscle in the arm and then provides a vibratory stimulus to that same muscle at a rate proportional to the obtained EMG signal. In our studies with children with dystonia, we were able to show that only vibration that was proportional to muscle activity was able to change muscle coordination patterns due to it providing a task-relevant stimulus (Liyanagamage et al., 2017). Vibratory feedback that was provided at a constant or random frequency provided no effect on muscle patterns. In my postdoctoral work, I examined the role of proprioception in producing accurate and coordinated movement using both kinematics and computer simulations of reaching movements. We found that in the absence of proprioception (due to a rare case of peripheral deafferentation), each hand behaves like an independent controller – the left hand makes large initial movement planning errors while the right hand makes large drifts at the end of movement (see Figure 3, Jayasinghe et al., 2020). This suggests that lateralized motor controllers cannot properly integrate information without sensory feedback. I am now extending my graduate and postdoctoral work in understanding the role of sensory feedback on motor control to answer questions related to the utility of augmented sensory feedback during cognitively challenging reaching tasks. Our overarching goal with conducting basic neuroscience research is to develop a foundation from which we can design robotic tools for movement rehabilitation that incorporate sensory feedback in a manner that does not increase the cognitive load on the individual.
REFERENCES
Bagesteiro, L. B. & Sainburg, R. L. Nondominant arm advantages in load compensation during rapid elbow joint movements. J. Neurophysiol. 90, 1503–1513 (2003).
Barker-Collo, S. & Feigin, V. The impact of neuropsychological deficits on functional stroke outcomes. Neuropsychol. Rev. 16, 53–64 (2006).
Lang, C. E., Bland, M. D., Bailey, R. R., Schaefer, S. Y. & Birkenmeier, R. L. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J. Hand Ther. 26, 104–115 (2013).
Lawrence, E. S. et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 32, 1279–1284 (2001).
Philipose, L. E., Alphs, H., Prabhakaran, V. & Hillis, A. E. Testing conclusions from functional imaging of working memory with data from acute stroke. Behav. Neurol. 18, 37–43 (2007).
Sainburg, R. L. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 142, 241–258 (2002).
Schaefer, S. Y., Mutha, P. K., Haaland, K. Y. & Sainburg, R. L. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb. Cortex 22, 1407–1419 (2012).