Smartphone-driven microfluidics for at-home diagnostics
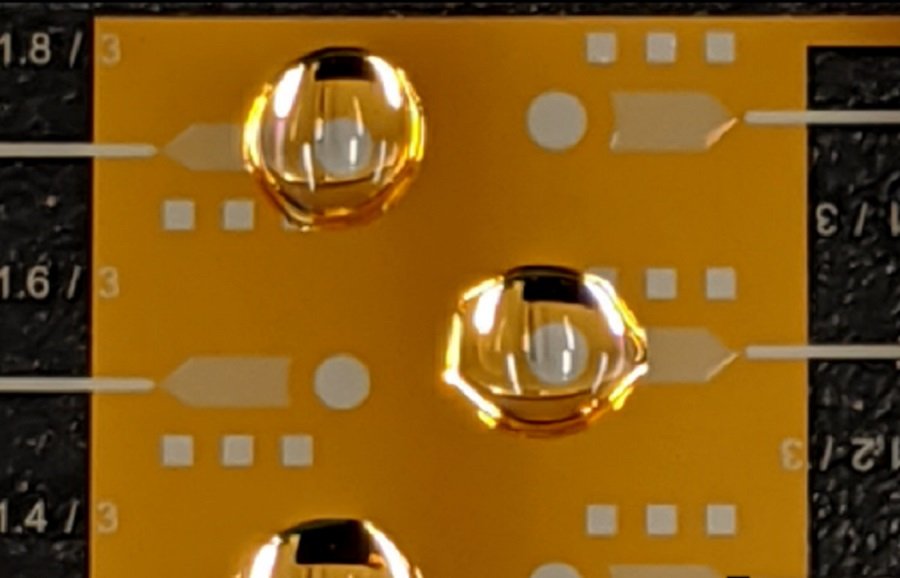
A team of researchers from ECE led by Distinguished McKnight University Professor and Sanford P. Bordeau Chair Professor Sang-Hyun Oh have developed a novel method to wirelessly actuate liquid microchannels using a smartphone. The development is significant for the wide range of applications it can have, particularly in the healthcare field, in its potential ability to increase mobility and accessibility of diagnostic technologies. The method is reported in a paper titled “Open-channel microfluidics via resonant wireless power transfer” in Nature Communications. Besides Oh and lead author Christopher Ertsgaard, the research team comprised researchers, Daehan Yoo, Peter Christenson, and Daniel Klemme.
Lead author Ertsgaard explains, "By utilizing a lower surface energy electrode geometry, we can actively manipulate liquid channels without pumps, tubing, or electrical cabling. This greatly simplifies microfluidic processing. For instance, in the case of physiological samples, you can maintain basic fluidic processing steps on a chip while removing the obstructing hardware that often frustrates or even limits laboratory setups. Moreover, considering the fact that it can be operated wirelessly, one can appreciate its user-friendly capability to bring fluidic diagnostics from the lab to your home or remote locations."
Active microfluidics with its several advantages has garnered interest and applications in diverse areas. However, despite the advantages of laboratory liquid processing, precise positioning and confinement with integrated sensors, low sample volumes, and portable sample processing, the technology has not taken off as expected. It remains limited in use because of the challenges it presents, namely, extreme surface tension (ST) as one scales down from a macro-level assembly meant for human handling to a densely packed micro scale of channels, tubes, pumps, and other components. The intricate assembly and expertise required to operate such systems has restricted their commercial use.
Prevailing techniques and their challenges
Electro-fluidics counters these challenges by doing away with the bulky equipment, and instead uses electric fields to position the liquid on the sensing component. Electrowetting on dielectric (EWOD) is one such example that has attracted a lot of interest, and has found commercial and scientific applications including biological assays, energy harvesting, and digital displays. However, it presents its own challenges as it manipulates at a macro-droplet scale. The method loses the advantages of microfluidics such as precision (in terms of liquid quantity and its placement), and other nuanced actions like localized chemical reactions and flow-based assays.
Electro-actuated capillary-driven microfluidics, a workaround, can counter the problem of precision by confining the sample with rigid microchannels. It uses electric fields to trigger capillary action at specified locations. While such systems confer micrometer-sized channels without the need for pumps, (ST) has proven to be an impediment to successful actuation of open-channels. Therefore, tubing and micro-assemblies of the rigid channels are still required, complicating laboratory setups and limiting its scalability for commercial manufacturing.
Other approaches exist that can remove intricate assembly, such as optically driven microfluidics and liquid dielectrophoresis (LDEP). Optically driven microfluidics uses the confined electric field of a laser source to arbitrarily extrude liquid, but is not scalable as it requires bulk optics. LDEP on the other hand answers the challenge by using radio frequency signals that can be generated using integrated circuits, but ST again poses a challenge and necessitates high operating voltages (between 50 to 200 VRMS).
Overall, while these existing techniques overcome some of the limitations of precision and placements in part, persistent challenges such as increased ST, intricate assembly, and large operating voltages have hindered the widespread use of the technology.
Breakthrough in open-channel microfluidics
Identifying the limiting factors (ST, and large operating voltages), the Oh research team have developed a technique that blends the advantages offered by both capillary-driven electrofluidics and LDEP. This new scheme is based on an efficient electrode geometry that diminishes the deleterious effects of surface tension on the fluid, while also addressing the problem of high voltage. Similar to the phenomenon of “legs” or “tears” that form along the glass of a full-bodied wine, known as the Gibbs-Marangoni effect. a local ST gradient is created, but by confined electrodes. These electrodes then drive the fluid as narrow “legs” or channels with micrometer precision.
The new microfluidic platform is particularly significant as it allows for active fluid transport of biological samples while also doing without external actuating components such as intricately fabricated enclosed channels, pumps or tubing. The team has achieved actuation of open-microchannels as narrow as 1 micrometer (µm) using voltages as low as 0.5VRMS. Their research also includes demonstrations of various applications of the new platform including protein labeling, particle filtering, and viral transport for infrared biosensing.
Emphasizing the significance of the team's work, Professor Oh explains, "In the wake of a global pandemic, now more than ever there is motivation to develop more sophisticated early detection technologies. We are excited to contribute this advancement to the field of microfluidics while simultaneously capitalizing on a ubiquitous power source and controller—the smartphone. Continuing to the future, our wireless, open-channel microfluidic platform can scale alongside the trajectory of Moore’s law to embrace emerging technologies."
The low operating voltage requirement is especially compelling: the value is so low that the scientists have successfully demonstrated the actuation of liquid microchannels using wireless power transfer and a near field communication (NFC) signal emitted by a smartphone. This is a particularly significant advantage in the healthcare context where portability and user-friendliness are much desired. A sample drop is simply placed on the chip and wireless interfacing actuates the channels without any interfering hardware. By integrating the system with point-of-care technologies, it can substantially improve aspects such as portability and accessibility, and thus support more advanced, at-home diagnostic technologies for the future.
Funding Acknowledgements: This work was supported by the NSF Graduate Research Fellowship for Christopher Ertsgaard and the Sanford P. Bordeau Endowed Chair in Electrical Engineering for Professor Oh.