Pollen Preparation Procedure
V. Stefanova
CSD Facility, Department of Earth Sciences, University of Minnesota
Introduction
Pollen analysis is the principal technique available for determining vegetation response to past terrestrial environmental change. The technique has been in use for nearly a century, initially as a method for investigating past climatic changes. More recently, the importance for vegetation change of processes such as human impact, successional change and other biotic and abiotic factors have been recognized. Pollen analysis can be used to examine these factors (Bennet and Willis, 2001).
Pollen grains are microscopic in diameter (most often between 10 and 70 μm) and due to the extremely resistant characteristic of the pollen grain wall, pollen grains can be well preserved in lake sediments, peat deposits, soil, and rocks. The unique morphological characteristic of the pollen grains make them easy to identify to species level. Through proper chemical treatment pollen grains and spores can be extracted from field samples, concentrated, and mounted for identification and quantification.
A basic procedure for the preparation of sediment samples for the analysis of pollen is presented here following Bennet and Willis (2001). It is set out diagrammatically in the table below. More details and some additional techniques are covered in Faegri & Iversen (1989).
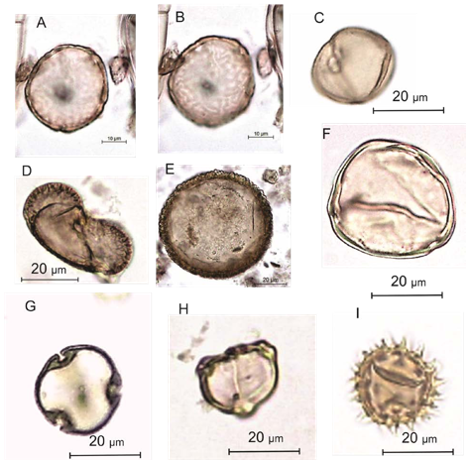
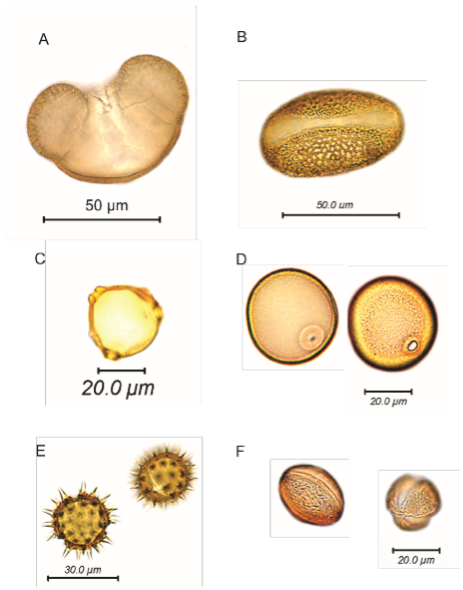
Images of sediment samples at different stages were taken to illustrate the processes in the pollen extraction procedure.
|
Treatment Step |
Result |
|---|---|
|
Step 1. Spike |
To estimate the concentration of pollen and spores in sediments |
|
Step 2. Hot 10% KOH |
Removes humic acids |
|
Step 3. Screening (160μm and 6μm) |
Removes coarse particles and clay or fine organic particles |
|
Step 4. Hot 10% HCl |
Removes carbonates |
|
Step 5. Hot 48% HF |
Removes silica and silicates |
|
Step 6. Hot 10% HCl |
Removes colloidal silica and silicates |
|
Step 7. Acetolysis |
Removes Polysaccharides (cellulose) |
|
Step 8. Dehydration |
Removes water |
|
Step 9. Mounting medium |
Silicone oil or glycerol |
Figure 1. Steps involved in sediment processing for fossil pollen
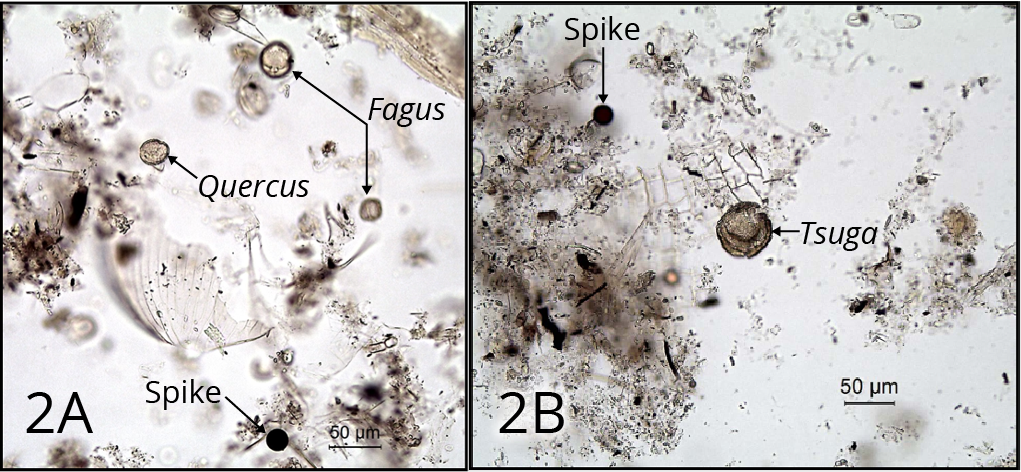
Step 1: Addition of spike
This is needed in order to obtain estimates of the concentration of pollen and spores in sediments. It must be the first stage, so that any losses of pollen and spores during the processing affect fossil and exotic pollen equally (Figure 2).
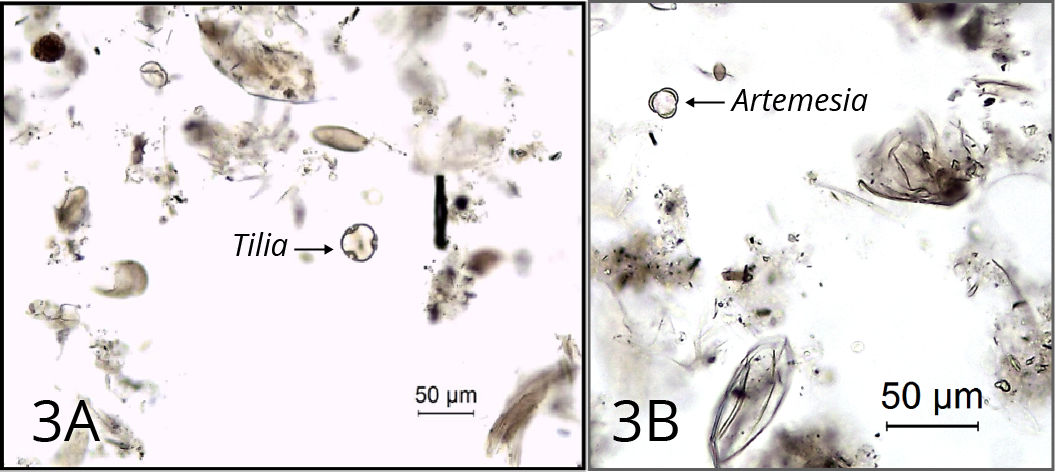
Step 2: Potassium hydroxide (KOH) treatment
This process removes ‘humic acids’ (unsaturated organic soil colloids) by bringing them into solution, and also disaggregates the sediment. The quantity of humic acid may be considerable in organic material that is highly decomposed (such as some peats) (Figure 3).
Step 3: Screening
Coarse screening (160 µm) the sample removes particles larger than most pollen or spores. The residue retained on the sieve can often usefully be examined for smaller identifiable macrofossils (such as trees bud-scales, small seeds of Potamogeton or Betula, conifer needles) (Figure 4). The fine screening (6 µm) removes fine organic particles and clay.

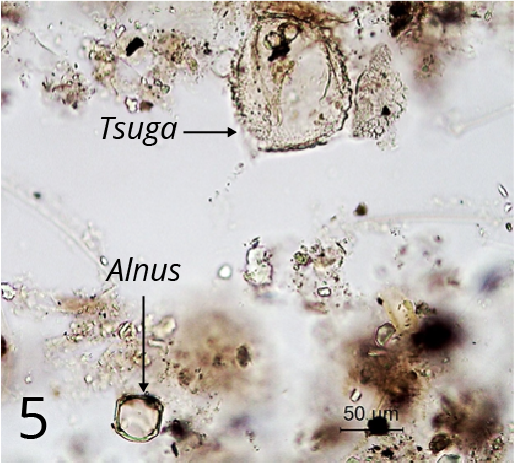
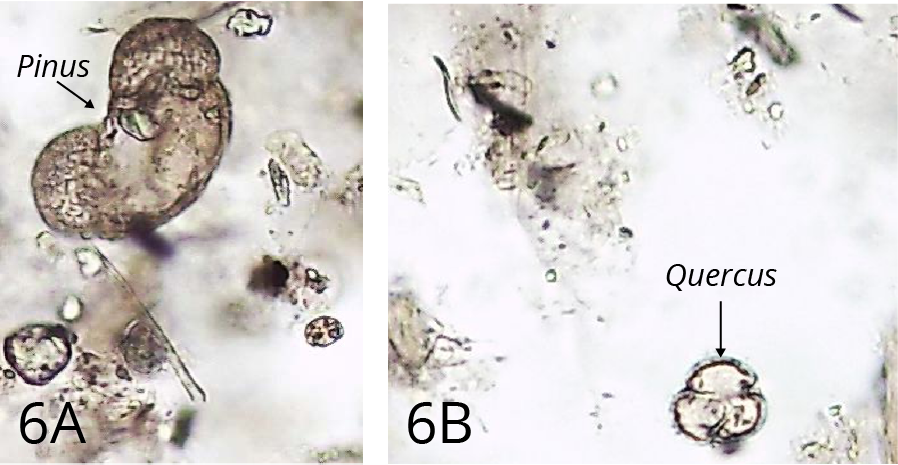
Step 4: Hydrochloric acid treatment
This process removes carbonates. The reaction products are calcium hydroxide, which is soluble, and carbon dioxide, which is released as a gas (often vigorously). Magnesium containing carbonate (which may occur in areas with dolomitic limestones) reacts in the same way, but much slower. If its presence is suspected, longer periods in hot HCl are needed (Figures 5 and 6)
Step 5: Hydrofluoric acid (HF) treatment
This stage removes silica and silicates.
Step 6: Hydrochloric (HCl) rinse
HCl step removes colloidal silica and silicofluorides. Depending on the composition of the sediment, this step may be at least as important as the HF step. The reaction between HF and some minerals may produce an insoluble white precipitate, consisting of fluorides.
Step 7: Acetolysis
This stage removes polysaccharides by hydrolysing the polymer chain into soluble monosaccharide units. Polysaccharides are present on the surface f the grain and in the cytoplasm, so removing them greatly facilitates viewing the grain. Polysaccharides such as cellulose may also be significant components of the sediment, so removing these helps concentrate the pollen in the residue (Figure 7).
Step 8: Dehydration
A series of increasingly concentrated alcohols and tertiary butyl alcohol remove water, which does not mix with the silicone oil mounting medium. If the water is not 100% removed, irreversible clumping always occurs.
Step 9: Mounting medium
A good mounting medium should have a refractive index close to sporopollenin (1.48) for sufficient contrast to see features of the grains. Silicone oil and glycerol are preferred mounting mediums (Figure 8).
References
Faegri, K. & J. Iversen, 1989. Textbook of Pollen Analysis (4th ed.). Wiley, Chichester, 328 p.
Bennet, K.D. and Willis, K. J. 2001. Pollen. In: J. P. Smol, H. B. Birks & W. M. Last (eds.) Tracking Environmental Change Using Lake Sediments. Volume 3: Terrestrial, Algal, and Siliceous Indicators. 5-32 p. Kluwer Academic Publishers, Dordrecht, The Netherlands.
