Catalysis, Separations & Reaction Engineering
Raw materials and intermediates are converted into a variety of chemical products including small platform molecules, intermediate ingredients, and larger fuel and polymeric products, among many other chemicals critical to society. Chemical conversion is inherently interdisciplinary due to the multiple time and length scales of reactor operation including molecular transformations of catalysis, mesoscale molecular transport of fluids in particles, devices, and membranes, and the operation scale of reactors, columns, and industrial beds. Research involves the fundamental understanding of the behavior of molecules on surfaces and in multi-phase systems, enabling the design and optimization of new technology for improved manufacturing processes with increased efficiency, sustainability, and economy.
Catalysis
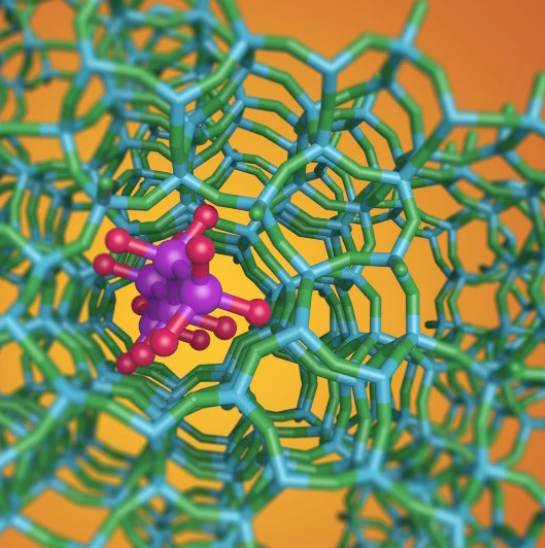
Conversion of molecules on active sites in porous materials or on surfaces of catalysts accelerates reactions and controls the competing pathways of chemistry. Fundamental understanding of the mechanisms and kinetics of catalytic turnover provides the basis for designing new materials that provide new capability for selective chemical conversion. Research combines experimental kinetics, spectroscopy, materials synthesis, computation, simulation, and machine learning to understand and optimize catalytic reactions.
Related Faculty and Research Groups:
Separations
Chemical processes convert feedstocks to targeted products often comprised of complex mixtures of molecules. Separations research aims to understand the thermodynamic and kinetic differences between molecular behavior for design of advanced separation technologies to more efficiently and sustainably separate molecules. Research combines computation, experimental kinetics and thermodynamics, simulation, and machine learning to understand and design advanced materials and operations to reduce energy and waste in separating mixtures.
Related Faculty and Research Groups:
Reaction Engineering
Reaction engineering aims to design reactors with controlled chemistry, catalysis, thermodynamics, and transport phenomena for optimal utilization of materials, sustainability, and economic output. Reactors and reaction processes are designed from the microscale to macroscale with varied design accounting for complex multi-phase or mixture behavior, often with integrated separation or energy transfer. Research combines simulation, computational design, and experimental optimization to understand and develop the next generation of chemical reactors.
Related Faculty and Research Groups:
Relevant Collaborative Partners and Core Facilities
Research related to catalysis, separations, and reaction engineering benefits from collaboration with industrial partners and university-wide centers and shared facilities including the Center for Programmable Energy Catalysis (CPEC), the Center for Sustainable Polymers (CSP), the UMN Characterization Facility (CharFac), and the Minnesota Nano Center (MNC).
Selected Publications
+
Catalysis, Mechanisms & Kinetics
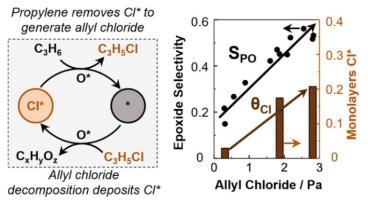
Selective propylene epoxidation over K-promoted Ag/CaCO3 features promotion by surface-bound chlorine adatoms (Cl*) deposited by trace alkyl chlorides co-fed continuously in a fashion similar to ethylene epoxidation over promoted Ag/α-Al2O3 catalysts Read More...
Related Faculty: Chris Bartel, Aditya Bhan, Paul Dauenhauer, Matthew Neurock, Kelsey Stoerzinger
+
Catalysis for Renewable Chemicals
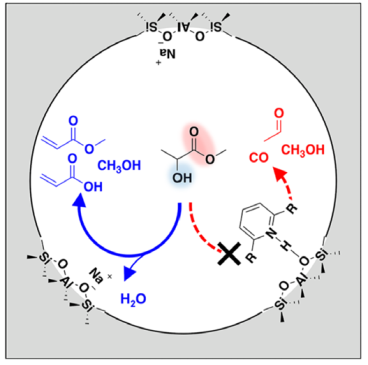
Dehydration of methyl lactate to acrylic acid and methyl acrylate was experimentally evaluated over a Na-FAU zeolite catalyst impregnated with multifunctional diamines. 1,2-Bis(4-pyridyl)ethane (12BPE) and 4,4′-trimethylenedipyridine (44TMDP), at a nominal loading of 40 wt % or two molecules per Na-FAU supercage, afforded a dehydration selectivity of 96 ± 3% over 2000 min time on stream Read More...
Related Faculty: Aditya Bhan, Paul Dauenhauer, Matthew Neurock
+
Computational Catalysis
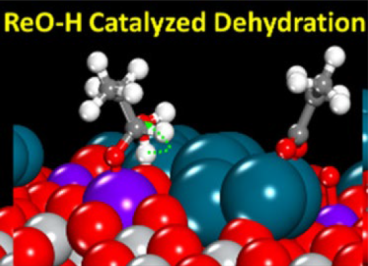
Silica- and titania-supported Pd, Re, and Pd-promoted Re catalysts were prepared by incipient wetness impregnation and characterized using X-ray diffraction and H2 chemisorption. The rate of catalytic reduction of propionic acid in H2 to predominantly form propanal and propanol over the Re-containing catalysts was insensitive to propionic acid pressure and 0.6 order in H2 pressure Read More...
Related Faculty: Matthew Neurock
+
Programmable Catalysis for Energy Carriers
Accelerating catalytic chemistry and tuning surface reactions require precise control of the electron density of metal atoms. In this work, nanoclusters of platinum were supported on a graphene sheet within a catalytic condenser device that facilitated electron or hole accumulation in the platinum active sites with negative or positive applied potential, respectively Read More...
Related Faculty: Aditya Bhan, Paul Dauenhauer, Dan Frisbie, Matthew Neurock, Kelsey Stoerzinger
+
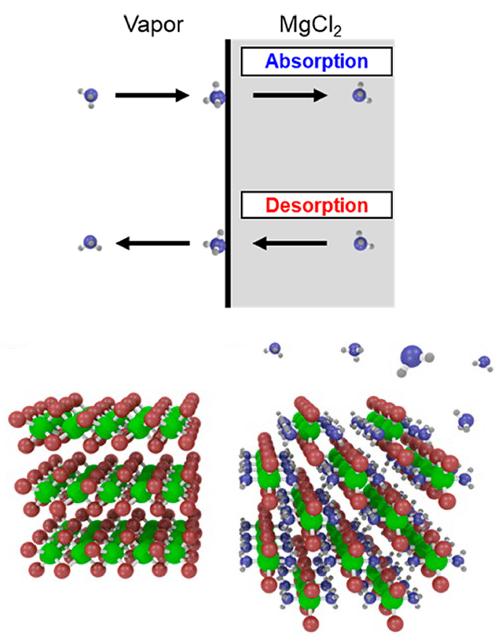
Separations for Renewable Energy Storage
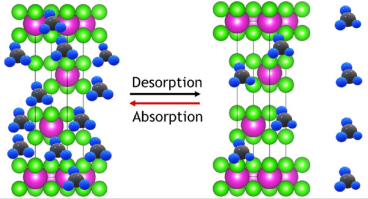
Ammonia made with hydrogen from sustainable wind energy can be separated from unreacted hydrogen and nitrogen by absorption in magnesium chloride. The absorption is rapid, but desorption can be slower. This work shows Read More...
Related Faculty: Paul Dauenhauer, Alon McCormick