Electrochemical Engineering
Electrochemical processes enable chemical reactions to be driven with electricity (and vice versa). Such processes find wide use, ranging from industrial metal production to biological sensors to energy storage devices. Electrochemical engineering is inherently interdisciplinary due to the wide range of phenomena at play, including electrical and mass transport, all coupled with chemical processes. Research involves the fundamental understanding of the (often competitive) reactions involved in these systems, and their relationship to system parameters and the materials through which ions and electrons flow. These efforts ultimately contribute to more efficient and long-lasting devices and greener processes driven by renewable electricity.
Electrochemical processes
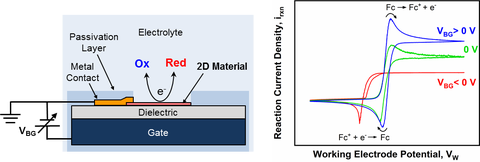
Electrochemical reactions can be used to sense molecules, deposit a material or change its properties, and store energy. Coupling of electrochemical processes is responsible for corrosion and other reactions. At the microscopic scale, these processes are driven by the arrangement of ions near (and sometimes within) a charged surface. Research combines materials synthesis, computation, experimental thermodynamics, spectroscopy, simulation, and machine learning to understand and design advanced materials and processes that operate efficiently.
Related Faculty and Research Groups
Electrocatalytic processes
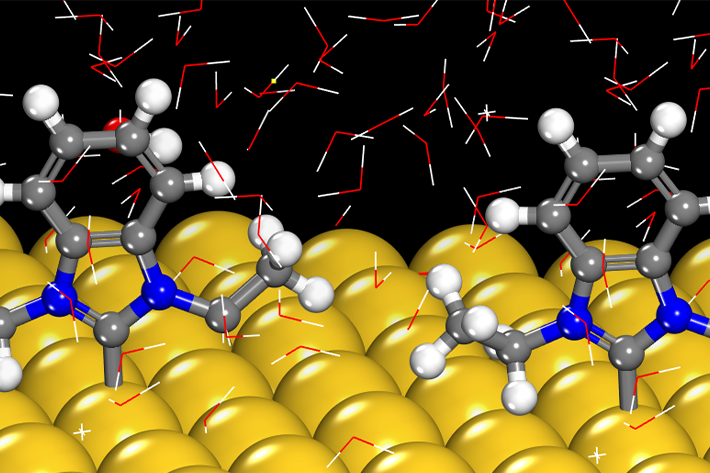
Electrocatalysts accelerate reactions and control competing reaction pathways. Fundamental understanding of the mechanisms and kinetics of catalytic turnover provides the basis for designing new materials that provide new capability for selective chemical conversion. Research combines experimental kinetics, spectroscopy, materials synthesis, computation, and simulation to understand and optimize catalytic reactions.
Related Faculty and Research Groups
Relevant Collaborative Partners and Core Facilities
Research related to electrochemical engineering benefits from collaboration with industrial partners and university-wide centers and shared facilities including the UMN Materials Research Science and Engineering Center (MRSEC), Molecular and Atomic EngineeRing of Interfacial Electrocatalytic Environments (MARIE), Center for Synthetic Organic Electrochemistry, Center for Programmable Energy Catalysis (CPEC), the UMN Characterization Facility (CharFac), and the Minnesota Nano Center (MNC).
Selected Publications
Programmable electrocatalysis for energy carriers
Hydrogen gas is a promising renewable energy storage medium when produced via water electrolysis, but this process is limited by the sluggish kinetics of the anodic oxygen evolution reaction (OER). Herein, we used a microkinetic model to investigate promoting the OER using programmable oxide catalysts (i.e., forced catalyst dynamics). Read more…
Related faculty: Dan Frisbie, Paul Dauenhauer, Kelsey Stoerzinger, Matthew Neurock
Electrochemical and electrocatalytic gating
Electrolyte-gate transistors are a powerful platform for control of material properties, spanning semiconducting behavior, insulator-metal transitions, superconductivity, magnetism, optical properties, etc. When applied to magnetic materials, for example, electrolyte-gate devices are promising for magnetoionics, wherein voltage-driven ionic motion enables low-power control of magnetic order and properties. Read more…
Related faculty: Dan Frisbie, Chris Leighton, Kelsey Stoerzinger, Bharat Jalan, Turan Birol
Machine learning for battery electrodes
Materials synthesis is a critical step in the development of energy storage technologies, from the first synthesis of newly predicted materials to the optimization of key properties for established materials. While the synthesis of solid state materials has traditionally relied on intuition-driven trial-and-error, computational approaches are now emerging to accelerate the identification of improved synthesis recipes. Read more…
Related faculty: Chris Bartel
Conversion of waste to energy carriers
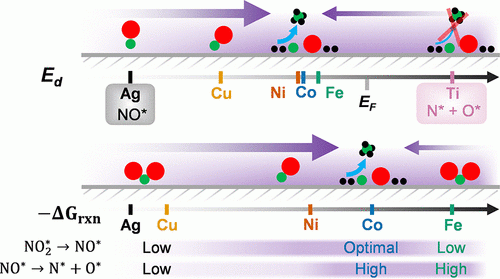
Electrocatalysis is a promising approach to convert waste nitrate to ammonia and help close the nitrogen cycle. This renewably powered ammonia production process sources hydrogen from water (as opposed to methane in the thermal Haber–Bosch process) but requires a delicate balance between a catalyst’s activity for the hydrogen evolution reaction (HER) and the nitrate reduction reaction (NO3RR) Read more…
Related Faculty: Matthew Neurock, Kelsey Stoerzinger
Corrosion
Alloys and oxidation-resistant coatings utilized in high-temperature applications can be degraded by aerosols that deposit onto surfaces during operation. Understanding how the deposit composition influences the hot corrosion mechanisms is essential to develop more durable materials. Read more…
Related Faculty: David Poerschke