A 3D model for finding new lung disease treatments

March 15, 2022 — BME Professor Dave Wood and team created a 3D platform for studying lung disease by recapitulating the tissue features of idiopathic pulmonary fibrosis (IPF).
Addressing barriers to new therapies
They published their work in APL Bioengineering in a study called, “A scalable 3D tissue culture pipeline to enable functional therapeutic screening for pulmonary fibrosis”. BME PhD student Katie Cummins is the study’s first author.
The 3D platform addresses a major challenge in IPF therapeutic development: A lack of high-fidelity models that account for the fibrotic microenvironment.
In the study, Prof. Wood and team demonstrated that normal lung fibroblasts encapsulated in collagen microspheres can be pushed toward an activated phenotype, treated with FDA-approved therapies, and their fibrotic function quantified using imaging assays (extracellular matrix deposition, contractile protein expression, and microenvironment compaction).
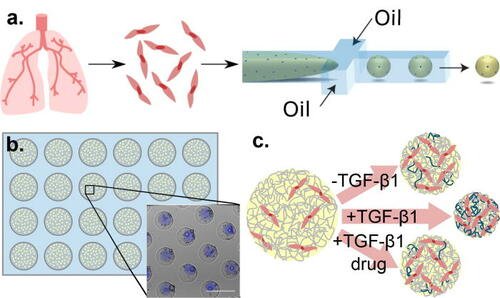
They further highlight the platform’s utility by showing that fibroblasts isolated from IPF patients’ lungs maintain fibrotic phenotypes and manifest reduced fibrotic function when treated with epigenetic modifiers.
Their platform enables enhanced screening, offering better predictability and fidelity than 2D systems along with better tractability and throughput than other 3D systems.
Potential for impact
The authors believe their IPF microtissue model can improve the IPF drug screening pipeline, which ultimately aids in therapeutic development.
Plus, the assays and methodologies outlined in the study should be of significant utility to investigations of other disease spaces in which 3D culture is important and tissue remodeling is prevalent — for example, other fibrotic disease, cancers that present as solid tumors, and diseases interfering with wound healing.