Feature tracking microfluidic analysis reveals differential roles of viscosity and friction in sickle cell blood

April 29, 2022 — Associate Professor Dave Wood and team developed a new method for analyzing patient-specific blood properties that can be applied to a wide range of hematological and vascular disorders. Characterizing blood flow rheology can help scientists better understand the pathophysiology of such disorders, but existing methods to measure these parameters are limited in their physiological relevance.
In a study published in Lab on a Chip, Prof. Wood and team presented their method, which combines microfluidic systems and powerful object-tracking computational technologies with mathematical modeling to separate the red blood cell flow profile into a bulk component and a wall component.
Using this framework to evaluate blood from patients with sickle cell disease, the research team:
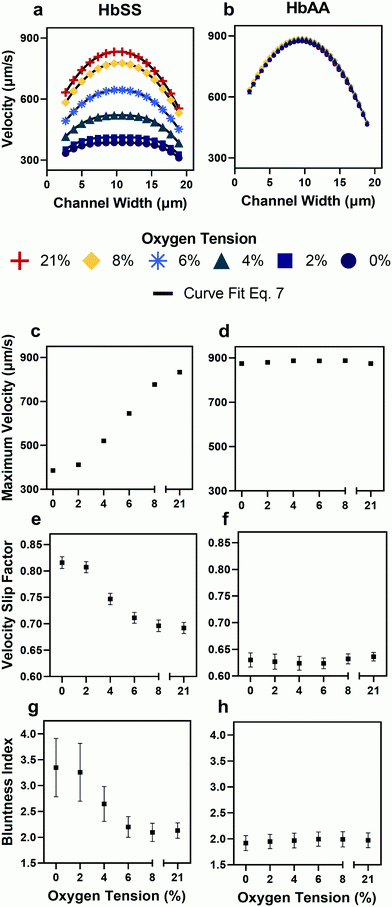
- Demonstrated that blood from patients with sickle cell disease exhibits elevated frictional and viscous resistances at all physiologic oxygen tensions.
- Found that the viscous resistance increases more rapidly than the frictional resistance as oxygen tension decreases, which may confound analyses that extract only flow velocities or overall flow resistances.
- Evaluated the impact of transfusion treatments on the components of the resistance, revealing patient variability in blood properties that may improve scientists’ understanding of the heterogeneity of clinical responses to such treatments.
Prof. Wood and team anticipate that, in the future, systems like theirs could potentially be used to cheaply and efficiently analyze the blood of patients with sickle cell disease. By combining these results with clinical outcomes, such analyses could determine vaso-occlusive risk and predict optimal transfusion ratios and treatment regimens that reduce pathology in sickle cell disease.