Past Seminars & Events
4th-Year Graduate Research Seminar Series: Session 3
Tuesday, Oct. 6, 2020, 9:30 a.m. through Tuesday, Oct. 6, 2020, 11 a.m.
Michael Dorantes at 9:30 a.m.
Adviser: Professor Connie Lu
Catalytic N2 to NH3 conversion by a tin-supported Iron complex
Yukun Cheng at 10 a.m.
Adviser: Professor Ian Tonks
Synthesis of Pentasubstituted 2-Aryl Pyrroles from Boryl and Stannyl Alkynes via One-Pot Sequential Ti-Catalyzed [2+2+1] Pyrrole Synthesis/Cross Coupling Reaction
Chase S. Abelson at 10:30 a.m.
Adviser: Professor Lawrence Que Jr.
A Highly Reactive High-Valent Iron-Oxo Oxidant Generated upon the Addition of Strong Acids to a FeIII-OOH Complex
Professor Sapna Sarupria
Monday, Oct. 5, 2020, 4 p.m. through Monday, Oct. 5, 2020, 5 p.m.
Via Zoom
Professor Sapna Sarupria
Department of Chemical & Biomolecular Engineering
Clemson University
Host: Professor Ilja Siepmann
Abstract
Nano-diving into the clouds: Uncovering molecular mechanisms of heterogeneous ice nucleation
The presence of particles such as dust and pollen affect cloud microphysics significantly through their effect on the state of water. These particles can hinder or accelerate the liquid-to-solid transition of water, and also affect the ice polymorph formed in the clouds. This indirectly cloud reflectivity, cloud lifetime, and precipitation rates. While a predominant phenomenon, the understanding of the surface factors that affect ice nucleation is minimal. In our research, we use molecular simulations to illuminate the pathways through which surface properties influence ice nucleation. Experiments cannot probe the length and time scales relevant to nucleation. While molecular simulations, in principle, can probe the length and time scales of nucleation, in practice nucleation is challenging to sample. Nucleation is often associated with large free energy barriers and thus, is difficult to sample in straightforward simulations. Advanced sampling techniques and other creative approaches are needed. In this talk, I will discuss the insights we have obtained on heterogeneous ice nucleation through studies of three surfaces – silver iodide, kaolinite and mica. I will also highlight the synergistic combination of experiments and simulations in understanding heterogeneous ice nucleation. I will introduce a recently developed method in our group that facilitates computational studies of heterogeneous nucleation. I will conclude by providing a perspective on the broader implications of our studies on interfacial phenomena and surface design.
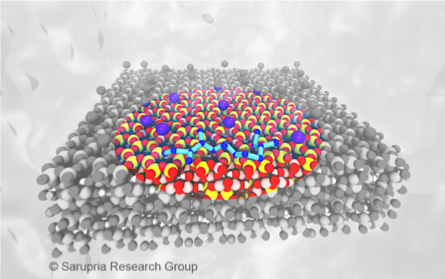
Professor Sarupria
Sapna Sarupria is an associate professor in Chemical and Biomolecular Engineering at Clemson University. She received her Master's degree from Texas A & M University where her thesis focused on thermodynamic modeling of clathrate hydrates of gas mixtures formed in the presence of electrolyte solutions. She obtained her doctorate from Rensselaer Polytechnic Institute, where she studied pressure effects on water-mediated interactions and proteins. She was a postdoctoral researcher in Princeton University and studied hydrate and ice nucleation using advanced path sampling techniques. She received the NSF CAREER award, ACS COMP Outstanding Junior Faculty Award and Clemson’s Board of Trustees Award of Excellence. She is an active member of Women in Chemical Engineering (WIC) and Computational and Molecular Science and Engineering Forum (CoMSEF) in AIChE.
Research
Professor Sarupria's research focuses on surface-driven phenomena. Current projects include heterogeneous ice nucleation, protein adsorption on surfaces and fouling on water purification membranes. The central theme in Sarupria group involves developing cutting-edge sampling techniques in molecular simulations and applying them in understand long standing problems in condensed matter. We recently developed novel transition path sampling methods and software to enable their large-scale implementation in HPC infrastructure. These methods will be used to study ice nucleation, and reactions in condensed phases including enzymatic reactions.
4th-Year Graduate Research Seminar Series: Session 2
Thursday, Oct. 1, 2020, 9:30 a.m. through Thursday, Oct. 1, 2020, 11 a.m.
Mengyuan Jin at 9:30 a.m.
Adviser: Professor Thomas Hoye
Untethered/Intermolecular” Hexadehydro-Diels–Alder (HDDA) Reaction of Alkynyl Borane Esters Mediated by Lewis-Pair Interaction
Stephanie R. Liffland at 10 a.m.
Adviser: Professor Marc Hillmyer
Mechanical Properties of Linear and Star Block Aliphatic Polyester Thermoplastic Elastomers
Ethan A. Gormong at 10:30 a.m.
Advisers: Professor Theresa M. Reineke & Professor Thomas R. Hoye
Adapting the Glaser-Hay Coupling toward Sugar-derived Poly(propargyl ether diynes)
4th-Year Graduate Research Seminar Series: Session 1
Tuesday, Sept. 29, 2020, 9:30 a.m. through Tuesday, Sept. 29, 2020, 11 a.m.
Claire E. Dingwell at 9:30 a.m.
Adviser: Professor Marc Hillmyer
Processable and Photocurable Epoxy-functional Polyolefin Prepolymers from Ring-Opening Metathesis Polymerization
Brendan J. Graziano at 10 a.m.
Adviser: Professor Connie Lu
Organometallic Reactivity of Nickel Complexes Bearing PAlP Pincer-Type Ligands
Rishad J. Dalal at 10:30 a.m.
Adviser: Professor Theresa Reineke
Cationic Bottlebrush Polymers for Nucleic Acid Delivery
Kevin Cole, Ph.D., Junliang Hao, Ph.D.
Monday, Sept. 28, 2020, 4 p.m. through Monday, Sept. 28, 2020, 5 p.m.
Kevin Cole, Ph.D.
Senior Research Scientist
Eli Lilly and Company
Junliang Hao, Ph.D.
Research Adviser, Discovery Chemistry
Eli Lilly and Company
Host: Nicholas Race
Discovery and development of mevidalen, a first-in-class positive allosteric modulator of the dopamine D1 receptor

Kevin Cole was born in St. Paul, MN, and received his Bachelor of Science in chemistry from the University of Minnesota in 1999. He stayed at Minnesota for graduate studies, earning a doctorate in 2005, working with Professor Richard Hsung developing novel methodologies to apply to natural product total synthesis. He then moved to The Scripps Research Institute in San Diego to work with Professor K.C. Nicolaou investigating complex natural product synthesis. In 2007, Cole joined the process development group at Eli Lilly in Indianapolis, IN. As a principal research scientist, Cole has primarily worked to develop new production routes for early–mid phase clinical assets and has utilized continuous processing to support the supply for a number of clinical assets.

Junliang Hao received his doctorate from University of Minnesota in 2002, and did his post-doctoral researches at The Scripps Research Institute and Harvard University. He started at Eli Lilly at Indianapolis in 2006, and has worked primarily on small molecule drug discovery. More recently, he has worked on RNA therapeutics. He currently holds the title of Research Adviser, and his research has contributed to several clinical candidates that have reached phase two clinical studies, one of which is the first-in-class D1PAM he will share in this seminar.
Professor Ellen Sletten
Thursday, Sept. 24, 2020, 9:45 a.m. through Thursday, Sept. 24, 2020, 11 a.m.
Via Zoom
Professor Ellen Sletten
Department of Chemistry & Biochemistry
University of California, Los Angeles
Host: Professor Mark Distefano
Abstract
Polymethine fluorophores for in vivo shortwave infrared imaging
Fluorescence imaging is a central tool for visualizing complex biological systems, yet the contrast and resolution attainable in vivo is limited by diffuse light originating from background and scattering at visible and near-infrared (NIR) wavelengths. Recently, the shortwave infrared region of the electromagnetic spectrum (SWIR, 1000 – 2000 nm) has emerged as an optimal region for in vivofluorescence imaging due to its minimal light scattering and low tissue autofluorescence compared to the NIR. While the SWIR demonstrates great promise, suitable materials are needed with emission at these low energies for the development of optical contrast agents. Our group develops biocompatible polymethine fluorophores for the shortwave infrared region. In 2017, we discovered a bright shortwave infrared emitter containing flavylium heterocycles that we deemed Flav7. Since that time, we have systematically investigated Flav7 using physical organic chemistry approaches and can now predictably tune the absorption and emission properties. These insights have lead to new SWIR fluorophores that enable multiplexed in vivo imaging and the fastest SWIR imaging to date.
Research
Professor Ellen Sletten's research group applies the fundamental principles of physical organic chemistry to create enhanced nanotherapeutics and diagnostics, new chemical tools to study living systems, and novel light-harvesting materials. The central theme of her group is the element fluorine, which imparts unique, orthogonal behavior to molecules and materials. Research within the group involves an interdisciplinary mix of organic synthesis, fluorous chemistry, chemical biology, self-assembly, polymer synthesis, photophysics, nanomedicine, and pharmacology.
Professor Sletten
Professor Sletten received her bachelor's degree from Stonehill College, and her doctorate at the University of California, Berkeley. Her thesis work involved the optimization and development of bioorthogonal chemistries and their subsequent applications in labeling living systems. She performed her post-doctoral studies at the Massachusetts Institute of Technology. She joined the faculty at UCLA in 2015.
Professor Julia Kalow
Tuesday, Sept. 22, 2020, 9:45 a.m. through Tuesday, Sept. 22, 2020, 11 a.m.
Via Zoom
Professor Julia Kalow
Department of Chemistry
Northwestern University
Host: Stephanie Liffland, president POLY/PMSE UMN Student Chapter
Abstract
Reactivity-property relationships in photocontrolled polymer networks
In polymer networks based on dynamic covalent bonds, changes in reactivity can be translated into macroscopic responses. Light offers precise, tunable, and noninvasive spatiotemporal control over molecular reactivity. The Kalow lab has designed crosslinks that allow us to tune the thermodynamics and kinetics of dynamic covalent bonds with light, including visible light, based on the conformation of an adjacent photoswitch. When incorporated into polymer networks, the mechanics can be tuned reversibly with light. I will discuss the molecular mechanism underlying these macroscopic changes, and their applications in biomaterials.
Research
Professor Julia Kalow engages in research at the interface of organic synthesis, polymer chemistry, and materials science through two distinct approaches: materials-inspired reaction discovery and reactivity-driven materials discovery. In the first, researchers develop new synthetic transformations that provide control over the molecular structure of 1- and 2-dimensional organic polymers. In the second, they use knowledge of organic reactivity to tune the properties and functions of soft materials. These efforts enable the study of structure-property relationships and materials optimization for targeted applications, including optoelectronics and magnetooptics, catalysis, sensing, and biomaterials.
Professor Kalow
Professor Kalow obtained her Bachelor of Arts degree at Columbia University, where she studied chemistry and creative writing. Following an internship in the medicinal chemistry department at Merck, she pursued graduate studies at Princeton University as a National Science Foundation predoctoral fellow. She developed asymmetric catalytic fluorinations using a latent source of HF and studied their mechanisms in detail; her work was recognized by an American Chemical Society Division of Organic Chemistry Graduate Fellowship Award. After completing her doctorate, she joined the Massachusetts Institute of Technology as a Ruth L. Kirschstein National Institutes of Health National Research Service Award postdoctoral fellow, studying the synthesis and self-assembly of novel architectures of conjugated block copolymers as well as responsive surfactant design for sensing applications. She started her independent career at Northwestern’s Department of Chemistry in July 2016.
Professor Annia Galano
Monday, Sept. 21, 2020, 4 p.m. through Monday, Sept. 21, 2020, 5 p.m.
Via Zoom
Professor Annia Galano
Department of Chemistry
Metropolitan University of Mexico
Mexico City, Mexico
Host: Professor Ilja Siepmann
QM-ORSA: An accurate computational protocol to explore the tip of the antioxidant iceberg
Chemical antioxidants are potential candidates to ameliorate the deleterious effects of oxidative stress-related diseases. However, both oxidative damage and antioxidant protection are complex and interrelated processes. That is why investigations on this field frequently focus on specific aspects of the whole phenomenon. Probably, the most widely explored aspect of chemical antioxidants is their ability to deactivate free radicals. The Quantum Mechanics-based Test for Overall Free Radical Scavenging Activity (QM-ORSA) is a computational protocol designed to be a reliable tool in the study of radical-molecule reactions in solution. It can be used to provide a universal and quantitative way of evaluating the free radical scavenging activity of chemical compounds. It provides a separated quantification of such activity in polar (aqueous) and non-polar (lipid) media. It includes two different scales for quantification: (i) absolute, based on overall rate coefficients; and (ii) relative, using Trolox as a reference antioxidant. QM-ORSA also allows identifying the main mechanisms of reaction involved in the free radical scavenging activity of chemical antioxidants and establishing the influence of pH on such an activity. The QM-ORSA protocol has been validated versus experimental results, and its uncertainties were proven to be no larger than those arising from experiments.
References:
- Annia Galano, Juan Raúl Alvarez-Idaboy “A Computational Methodology for Accurate Predictions of Rate Constants in Solution: Application to the Assessment of Primary Antioxidant Activity” J. Comput. Chem. 2013, 34 (28), 2430–2445.
- Annia Galano, Gloria Mazzone, Ruslán Alvarez-Diduk, Tiziana Marino, J. Raúl Alvarez-Idaboy, Nino Russo. “Food Antioxidants: Chemical Insights at the Molecular Level” Annu. Rev. Food Sci. Technol. 2016, 7, 335–352.
- Annia Galano, Juan Raúl Alvarez-Idaboy “Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow?” Int. J. Quantum Chem. 2019, 119, e25665 (23 páginas).
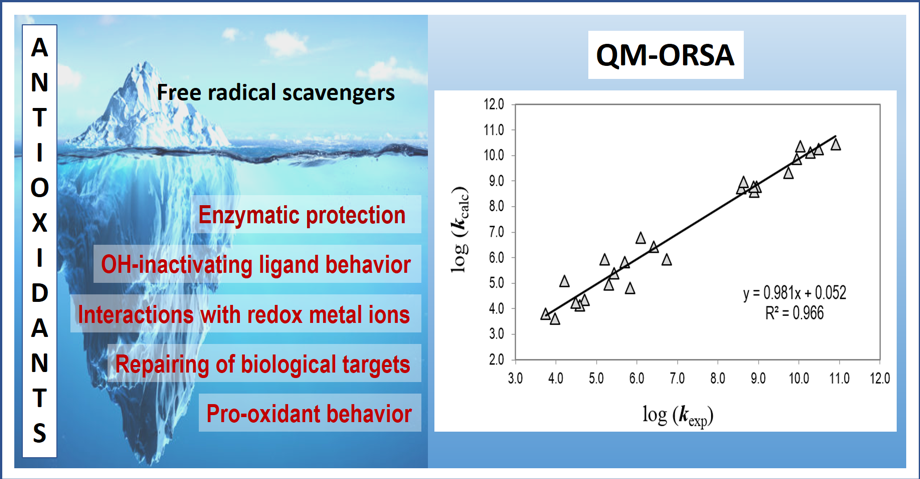
Research
Professor Annia Galano’s research group applies computational chemistry to the investigation of physicochemical insights directly related to atmospheric pollution, carbon-based nanomaterials, oxidative stress, and antioxidant activity. The current central themes of her group are:
- The elucidation of the chemical behavior of antioxidants and the relative efficiency for that purpose of a large variety of chemical compounds.
- The systematic design of new antioxidants with multifunctional behavior and possible neuro-protective effects.
Her group is involved in interdisciplinary research that mixes theoretical chemistry, organic synthesis, analytical chemistry, chemical biology, and medicinal chemistry.
Professor Galano received her bachelor’s degree and her doctorate at Havana University, Cuba. She performed postdoctoral studies at the Mexican Institute of Petroleum and at the Metropolitan Autonomous University (UAM, according to its Spanish acronym). She had research stays at Uppsala University and Calabria University and joined the faculty at the UAM in 2008.
Professor Erin E. Carlson
Thursday, Sept. 17, 2020, 9:45 a.m. through Thursday, Sept. 17, 2020, 11 a.m.
Via Zoom
Professor Erin E. Carlson
Department of Chemistry
University of Minnesota
Host: Professor Mark Distefano
Abstract
Chemical Microbiology: Tools for the Translation and Control of Bacterial Behavior
With the “age of antibiotics” in the 1940s, many believed that we had conquered pathogenic microbes. However, it quickly became apparent that the ability of bacteria to evolve resistance had been sorely underestimated. Today, infectious diseases are the leading cause of death in low-income countries and the death toll is projected to outpace cancer in the United States by 2050. To address this mounting challenge, our group is uniting tools from chemistry and biology to explore, exploit, and control microbial behavior.
Microbes adapt to ever changing environments while cooperating with and defending against numerous species, invading hosts, and thriving over a vast range of conditions. This success stems from their ability to sense and respond to diverse environmental cues, including trace nutrients and signals from neighboring biota. Given the extraordinary ability of microbes to respond to myriad stimuli, the focus of my research program is to interrupt bacterial signaling processes and control bacterial actions, with particular focus on the expression of virulence and pathogenesis factors that lead to significant morbidity and mortality. This strategy is in direct contrast to traditional antimicrobial discovery efforts that aim to simply kill bacteria, which results in the rapid evolution of resistance. This seminar will touch on four intersecting research areas to generate a deeper understanding of how to manipulate bacterial behavior and eliminate infectious disease:
- Development of chemical tools for the profiling and inhibition of histidine kinases, a ubiquitous class of signal transduction proteins in bacteria. Our inhibitors possess anti-virulence activity in multiple high-priority pathogens, including Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus.
- Design of chemical probes to expand our understanding of the penicillin-binding proteins, crucial players in bacterial cell wall biosynthesis and targets of the most widely used class of antibiotics, the β-lactams. Our probes enable the selective investigation of each PBP isoform, with focus on spatial mapping of their catalytic function.
- Assessment of the effects of engineered nanoparticles on biomolecules and microbes. We have generated the first evidence of the evolution of permanent bacterial resistance to complex metal oxide nanoparticles.
- Development of strategies to explore the molecular language, natural products, used by bacteria to respond to environmental cues through mass spectrometry and chemoselective reagents. These tools enable molecule discovery and characterization of secondary metabolites from myriad sources.
Professor Carlson
Professor Carlson earned her bachelor's degree from St. Olaf College and her doctorate from the University of Wisconsin. She was a post-doctoral researcher at The Scripps Research Institute before joining the faculty at Indiana University in 2008. In the summer of 2014, she joined the faculty in the Chemistry Department at the University of Minnesota, and was appointed as a graduate faculty member of the Department of Medicinal Chemistry, the Department of Biochemistry, Molecular Biology and Biophysics, and the graduate program in Biomedical Informatics and Computational Biology.
Since the start of her independent career, Carlson has won numerous awards, including being named a Presidential Early Career Awards for Scientists and Engineers (PECASE) recipient, a Pew Biomedical Scholar, the NIH Director’s New Innovator Award, the Indiana University Outstanding Junior Faculty Award, the NSF CAREER Award, the Cottrell Scholar Award and was named a Sloan Research Fellow and an Indiana University Dean's Fellow. Professor Carlson has been highlighted in several videos including one produced by NBC in their Science Behind The News series, a fast-paced video series supported by the National Science Foundation. This piece was one of five videos highlighting work funded by NSF's Directorate for Mathematical and Physical Sciences. She was also featured in a "Brilliant Minds" video. Carlson was also named one of "Tomorrow's PIs" in the sixth annual issue of Genome Technology and received the Outstanding Postdoctoral Mentor Award from the University of Minnesota Postdoctoral Association in 2017.
Professor Joseph Topczewski
Tuesday, Sept. 15, 2020, 9:45 a.m. through Tuesday, Sept. 15, 2020, 11 a.m.
Via Zoom
Professor Joseph J. Topczewski
Department of Chemistry
University of Minnesota
Host: Professor Steven Kass
Abstract
Azides and heterocycles: Fun with dynamic chemistry and reactive intermediates
Chiral amines and heterocycles are ubiquitous motifs in chemical synthesis. The efficient synthesis of certain chiral amines and heterocycles remain a tremendous challenge. Presented herein is an unusual approach to chiral amine synthesis that utilizes the spontaneous rearrangement of allylic azides (Winstein Rearrangement). One component of the equilibrating mixture can be selectively trapped, establishing the amine bearing stereocenter. However, accomplishing this in practice requires high levels of selectivity and the simultaneous application of numerous control elements. Approaches to enabling selectivity will be described along with synthetic applications of the reactions, including in the synthesis of heterocyclic products.
Research
Professor Joseph Topczewski's research lab aims to invent transformative synthetic methods to reduce chemical waste generation, while simultaneously redefining how chemists approach making medicines. His researcher discovers and optimizes innovative synthetic methods to address this 21st century challenge. Superior methods i) prevent unnecessary resource consumption, ii) minimize the generation of chemical waste, and iii) provide access to new chemical space. New chemical space contains molecular diversity with unknown potential. Current research focuses on i) enhancing chiral amine synthesis, ii) discovering salt-free coupling reactions, and iii) improving access to bromodomain inhibitors. These focus areas address specific longstanding synthetic challenges. The methods provide unique access to untapped chemical space, which researchers are exploring through collaboration for medicinal chemistry applications.
Professor Topczewski
Professor Joseph Topczewski earned his bachelor's degree from the University of Wisconsin at Parkside, and his doctorate from the University of Iowa. He was a post-doctoral research at the University of Iowa and Michigan before joining the Department of Chemistry at the University of Minnesota in 2015.