Professor Jonathan R. Nitschke
Kolthoff Lecture #3
Professor Jonathan R. Nitschke
Department of Chemistry
University of Cambridge, UK
Host: Professor Valerie Pierre
Material through Subcomponent Self-Assembly: Catalysts, Sensors, and Polymers
“Subcomponent self-assembly”[1] describes the formation of complex structures from simpler building blocks through the concomitant formation of coordinative (N→Metal) and dynamic-covalent imine (N=C) bonds. Some of the structures formed using this technique can serve as useful materials. Capsules can act as catalysts,[2] and they can sense and report the presence of a guest through fluorescence[3] or magnetism.[4] Double-helical metal-organic polymers[5] also show potentially useful properties as optoelectronic materials and memristors.
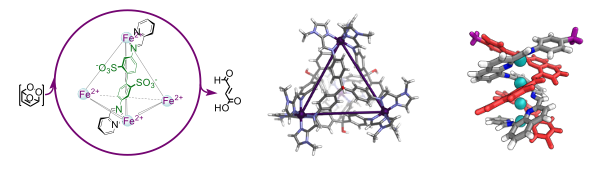
Figure 1. From left to right: catalytic transformation of a high-energy cyclic intermediate into a specific open-chain product;[2c] a spin-crossover cage that can magnetically sense guests;[4] a CuI4 model compound[6] for a series of copper-containing conjugated metallopolymers.[7]
[1] J. R. Nitschke, Acc. Chem. Res. 2007, 40, 103-112.
[2] (a) Z. Lu, R. Lavendomme, O. Burghaus, J. R. Nitschke, Angew. Chem. Int. Ed. 2019, 58, 9073-9077; (b) P. P. Neelakandan, A. Jiménez, J. D. Thoburn, J. R. Nitschke, Angew. Chem. Int. Ed. 2015, 54, 14378-14382; (c) A. G. Salles, S. Zarra, R. M. Turner, J. R. Nitschke, J. Am. Chem. Soc. 2013, 135, 17052-17059.
[3] A. J. Plajer, E. G. Percastegui, M. Santella, F. J. Rizzuto, Q. Gan, B. W. Laursen, J. R. Nitschke, Angew. Chem., Int. Ed. 2019, 58, 4200-4204.
[4] R. A. Bilbeisi, S. Zarra, H. L. C. Feltham, G. N. L. Jameson, J. K. Clegg, S. Brooker, J. R. Nitschke, Chem. Eur. J. 2013, 19, 8058-8062.
[5] J. L. Greenfield, J. R. Nitschke, Acc. Chem. Res. 2022, 55, 391-[391-401.
[6] J. L. Greenfield, F. J. Rizzuto, I. Goldberga, J. Nitschke, Angew. Chem., Int. Ed. 2017, 56, 7541-7545.
[7] J. L. Greenfield, D. Di Nuzzo, E. W. Evans, S. P. Senanayak, S. Schott, J. T. Deacon, A. Peugeot, W. K. Myers, H. Sirringhaus, R. H. Friend, J. R. Nitschke, Adv. Mater. 2021, 33, 2100403.
Jonathan R. Nitschke
Jonathan Nitschke received his bachelor’s degree from Williams College (USA) in 1995 and his doctorate from the University of California, Berkeley in 2001 under the supervision of T. Don Tilley. He then undertook postdoctoral studies with Jean-Marie Lehn in Strasbourg under the auspices of a US NSF fellowship, and in 2003 he started his independent research career as a Maître-assistant (fixed-term independent PI) in the Organic Chemistry Department of the University of Geneva. In 2007 he was appointed University Lecturer at Cambridge, where he now holds a Professorship. He is the recipient of the Izatt-Christensen Award in Supramolecular chemistry (2022), a Wolfson Research Merit Award of the UK Royal Society (2017), the International Award for Creative Work of the Japan Society of Coordination Chemistry (2016), the Bob Hay Lectureship of the Royal Society of Chemistry (2013), the Cram Lehn Pedersen Prize in Supramolecular Chemistry (2012), the Corday-Morgan Prize of the Royal Society of Chemistry (2011), the Dalton Transactions European/African Lectureship (2011), the Werner Prize of the Swiss Chemical Society (2007) and the European Young Chemist Award at the first EuCheMS Congress (2006). He won an ERC Starting Grant (2011-2016) and an ERC Advanced Grant (2017-2021). His research program investigates the self-assembly of complex, functional structures from simple molecular precursors and metal ions.
Kolthoff Lectureship in Chemistry
Izaak Maurits Kolthoff was born on February 11, 1894, in Almelo, Holland. He died on March 4, 1993, in St. Paul, Minnesota. In 1911, he entered the University of Utrecht, Holland. He published his first paper on acid titrations in 1915. On the basis of his world-renowned reputation, he was invited to join the faculty of the University of Minnesota’s Department of Chemistry in 1927. By the time of his retirement from the University in 1962, he had published approximately 800 papers. He continued to publish approximately 150 more papers until his health failed. His research, covering approximately a dozen areas of chemistry, was recognized by many medals and memberships in learned societies throughout the world, including the National Academy of Sciences and the Nichols Medal of the American Chemical Society. Best known to the general public is his work on synthetic rubber. During World War II, the government established a comprehensive research program at major industrial companies and several universities, including Minnesota. Kolthoff quickly assembled a large research group and made major contributions to the program. Many of Kolthoff’s graduate students went on to successful careers in industry and academic life and, in turn, trained many more. In 1982, it was estimated that approximately 1,100 Ph.D. holders could trace their scientific roots to Kolthoff. When the American Chemical Society inaugurated an award for excellence in 1983, he was the first recipient.
