CEGE leads $11 million project to advance mineral carbon storage

The University of Minnesota Twin Cities is the lead institution on a project that received $10.95 million over four years from the U.S. Department of Energy to host a new Energy Frontier Research Center (EFRC)—the Center for Interacting Geo-processes in Mineral Carbon Storage. The EFRC will bring together engineers and scientists from five internationally renowned organizations to study a promising technology for permanent solid storage of carbon dioxide (CO2) in geologic formations.
University of Minnesota Awarded Two New Energy Frontier Research Centers
One method of storing CO2 in the subsurface is to pump supercritical CO2 into porous rock that is capped by an impervious rock. This method, however, cannot guarantee that the carbon dioxide will stay sequestered in the reservoir. Indeed, if the integrity of the cap rock cannot be maintained, the CO2 could be released again into the atmosphere.
Recent scientific studies have uncovered a more stable means of storing CO2 underground by using carbon mineralization, or the process of converting CO2 into a carbon-based mineral. When CO2 comes into contact with a mafic or ultramafic rock, that is, rock rich in magnesium or calcium like basalt or peridotite, a chemical reaction produces solid carbonate minerals, locking in the CO2. This process has been observed to occur naturally in peridotite, and pilot studies have been implemented in basalt, but the hydro-mechanical interactions are not well understood. Due to the abundance of mafic and ultramafic rocks in the Earth’s subsurface—approximately 10% of the continents and a majority of the seafloor—this process provides a natural pathway by which CO2 can be permanently stored.
The rate of this process in nature is far too slow, however, to be effective in reducing anthropogenic emissions of CO2; an estimate from 1990, suggests a time of 70,000 years to remove the CO2 inventory from the atmosphere (Seifritz, 1990). However, emerging evidence shows that engineering this process has the potential of significantly increasing, by orders of magnitude, the rate of CO2 removal.
The new Center for Interacting Geo-processes in Mineral Carbon Storage at the University of Minnesota aims to further study carbon mineralization and ultimately engineer conditions that can make the process efficient. If successful, the work from these University of Minnesota engineers and scientists, along with colleagues at the University of Southampton, Georgia Institute of Technology, Northwestern University, and Los Alamos National Laboratory, has the potential to make this process widely applicable and even reverse some effects of climate change. The researchers will work to develop tools and methods for modeling this process and determining how much CO2 can be stored within a specific rock formation.
We are excited about this project because it will set us up, we hope, to make a significant impact on a critical societal issue: climate change,” said Emmanuel Detournay, project director and Bennett Professor of Rock Mechanics in the Department of Civil, Environmental, and Geo- Engineering. “It is appropriate for the Center to be housed at the University of Minnesota because of our expertise in geomechanics.”
Other faculty from the University of Minnesota include Peter Kang from the Department of Earth and Environmental Sciences, and Bojan Guzina, Joseph Labuz, Jia-Liang Le, Sonia Mogilevskaya, and Vaughan Voller from the Department of Civil, Environmental, and Geo- Engineering.
To make CO2 mineralization efficient requires
- Maximizing the reaction kinetics by dissolving CO2 in water and adjusting pressure and temperature.
- Increasing the rock surface available for reaction by pumping (injecting) the CO2 mixture through the fracture networks (natural or engineered) in the subsurface rock mass.
- Creating self-sustaining, reaction-driven cracking pathways by which the CO2 mixture can progressively advance from the fracture network into and through the bulk of the rock mass.
- Monitoring the progress of the mineralization via remote sensing, for example, seismic imaging.
These actions are very much in the realm of the unknowns. Although the researchers have an inkling of the elements that need to be characterized and adjusted to achieve feasible operation, there are however, extensive areas where a complete knowledge of engineered CO2 mineralization process in mafic and ultramafic rock is lacking. The key gap revolves around fundamental understanding of fluid flow and reactive transport in a fractured, multi-scale system where the permeability, porosity, pore space geometry, and available surface area are continually changing through time.
The operational problems occur at three distinct levels (see figure):
- Fracture network scale (>1m) forming the main arteries for moving a CO2-laden charge through the subsurface rock mass
- Fracture-porous medium system scale (0.1–1m) targeting individual links within the network, from which the charge can infiltrate into the rock mass
- Porous medium scale (< 0.1m) in the rock mass, where the bulk of the mineralization will need to occur
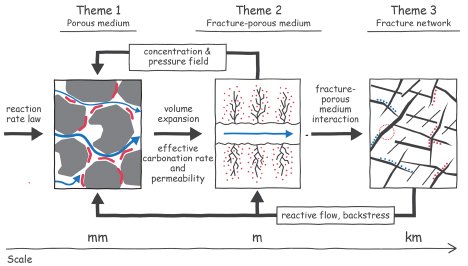
The schematic illustrates the scales and processes of the domains and the connections between them. The connections include research products and information that are passed from the small scales to larger scale and, also, from the large scales to smaller. Also, each of these research themes is not restricted to a single knowledge domain. The threads of geomechanics, geochemistry, porous media transport, and sensing technology are tightly woven through each research theme. The intimate coupling and integration are necessary for the researchers to answer the three fundamental overarching questions:
- What are the key factors and processes that determine the CO2 mineralization rates (mass/time) in mafic and ultramafic rock masses?
- How do these factors and processes depend on the host rock: mafic rocks (e.g., basalt) vs. ultramafic rocks (e.g., peridotite)?
- Once these factors and processes are identified and understood, how can the resulting models be deployed to generate hypotheses that can be tested at the scale of field operations?
A multi-disciplinary, multi-institutional Center is the best way to approach such a complex issue. Mineral carbon storage, the focus of the Center research has great potential to outpace the anthropogenic emissions of CO2 and even reverse some effects of climate change.