Bhagi-Damodaran group researchers discover how electrostatics control active site assembly and reactivity in iron enzymes that directly halogenate small molecules
MINNEAPOLIS / ST. PAUL (11/7/2023) – McKnight Land-Grant Professor Ambika Bhagi-Damodaran and her research group are making strides in sustainable enzyme research. Enzymes are specialized proteins – molecular tools – composed of amino acid sequences that perform complex chemical transformations across all forms of life. Unlike traditional methods of chemical synthesis that typically require toxic components and harsh conditions, enzymes are largely recyclable and perform their reactions in water under mild and environmentally-friendly conditions. Consequently, utilizing enzymes in the synthesis of industrially-relevant chemical compounds has the potential to substantially minimize the production of hazardous waste.
Direct and selective halogenation of aliphatic C-H bonds is a highly sought-after reaction in organic chemical synthesis. Halogenated molecules have extensive uses ranging from pharmaceutically active compounds to artificial sweeteners, in addition to being commonly used as synthetic intermediates for a variety of other chemical products. However, few small molecule catalysts can perform these halogenation reactions and, even when they do, they exhibit low selectivity. Iron-containing halogenases represent a class of enzymes able to perform selective halogenation through use of their mononuclear iron center and other cofactors.
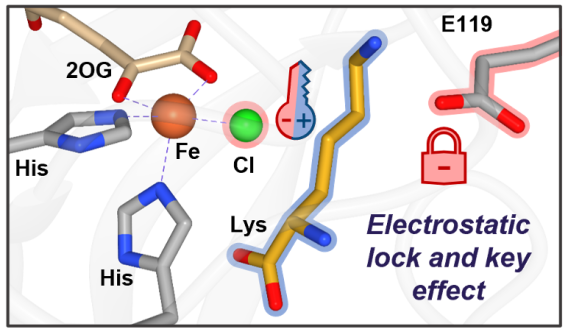
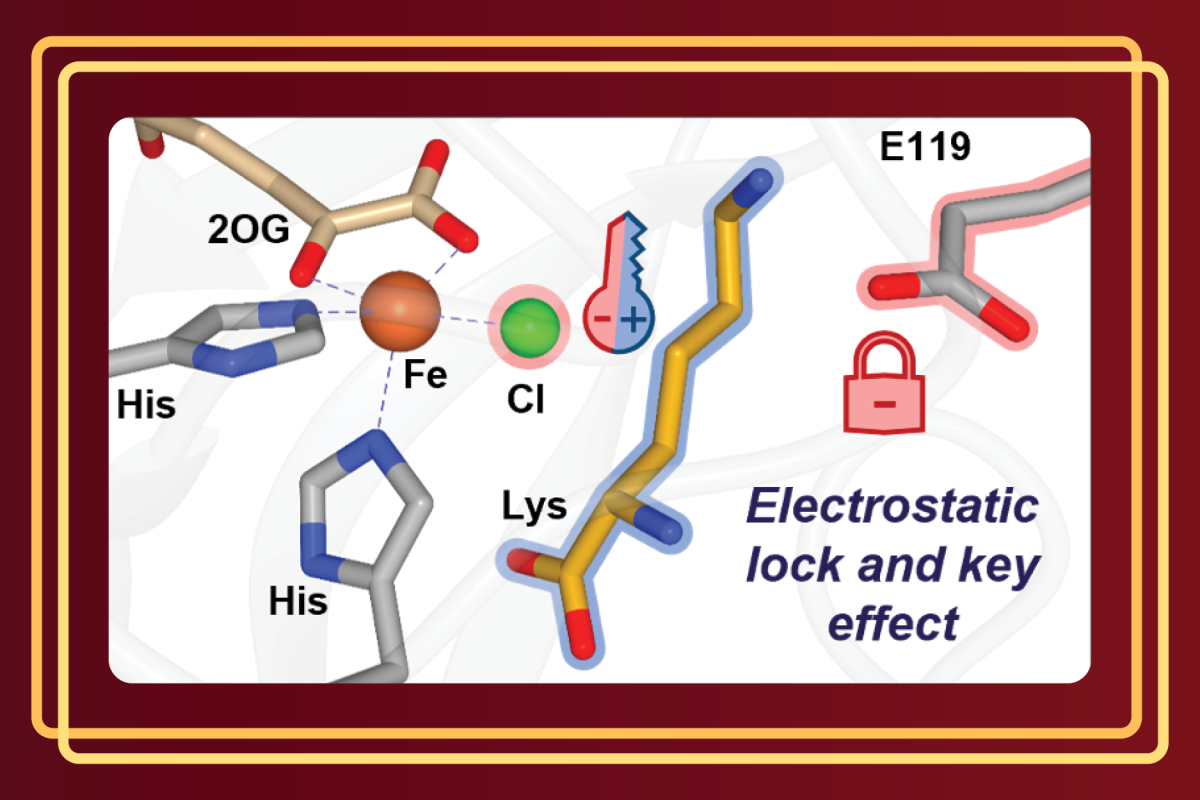
Researchers in the Bhagi-Damodaran group at the University of Minnesota have unraveled the role that electrostatics play in controlling active site assembly and reactivity of lysine-halogenating iron enzymes, BesD and HalB. Chemically modified amino acids like chlorinated lysine are often used as building blocks in pharmaceuticals, making the optimization of this family of iron halogenases commercially significant. To function properly, iron halogenases must facilitate the binding of both halide (chloride or bromide) and the substrate to the active site, in this case lysine. In their recent ACS Catalysis publication, Smithwick et. al. show that in BesD or HalB, the binding of either the halide or lysine is essentially non-existent until both are simultaneously present. Through a combination of computational and biochemical techniques, a negatively charged glutamate amino acid residue in the enzyme’s active site was shown to electrostatically enforce synergistic binding between the halide and the positively charged amino acid lysine. When the glutamate was mutated to an alternate amino acid that lacked this negative charge, the lysine-free binding affinity of chloride increased by 14-fold. Removing the dependency on lysine for chloride to bind will be an important step to facilitate access to a broader range of substrates and expand the industrial viability of this already promising family of enzymes.
Overall, the work by the Bhagi-Damodaran lab highlights the important role electrostatics play in substrate and anion recognition in BesD-like iron halogenases and illuminates concepts important for future biocatalytic engineering campaigns. “Understanding the mechanism by which enzymes regulate reactivity and function will enable scientists to better leverage the catalytic potential of enzymes to solve real world synthetic problems, leading to a cleaner and more sustainable future,” lead author of the article and chemistry graduate student Elizabeth Smithwick says.
The Bhagi-Damodaran group is made up of 11 researchers, graduate students, and undergraduate students. The group’s work is centered on the fundamentals of biological, inorganic, medicinal, and computational chemistry to gain insights into structure and function of metalloproteins and change their landscape in energy, catalysis, and medicine. Since joining the University of Minnesota Department of Chemistry in Fall 2018, Prof. Bhagi-Damodaran has been recognized numerous times for her research. Her recent awards include the 3M Non-Tenured Faculty Award (2020), the NIH Maximizing Investigator's Research Award (MIRA) (2020), the NSF CAREER Award (2021), the UMN McKnight Land-Grant Professorship (2023), and the Cottrell Scholar Award (2023).
Want to learn more about the Bhagi-Damodaran group’s enzyme chemistry research?
- Understanding ATP binding to DosS catalytic domain with a short ATP-lid. Larson GW, Windsor P.K., Smithwick E., Shi K., Aihara H., Damodaran, A.R., Bhagi-Damodaran A., Biochemistry 2023. https://doi.org/10.1021/acs.biochem.3c00306
- Metalloprotein enabled redox signal transduction in microbes. Williams M.T., Yee E., Larson G.W., Apiche E.A., Damodaran A.R., Bhagi-Damodaran A. Curr. Opin. Chem. Biol. (2023) https://doi.org/10.1016/j.cbpa.2023.102331
- Role of a secondary coordination sphere residue in halogenation catalysis of non-heme iron enzymes. Wilson R. H., Chatterjee S., Smithwick E. R. , Dalluge J. J., Bhagi-Damodaran A. ACS Catalysis 12(17), 10913 (2022) https://pubs.acs.org/doi/10.1021/acscatal.2c00954
- Integrating UV-Vis Spectroscopy and Oxygen Optode for Accurate Determination of Oxygen Affinity of Proteins. Damodaran A. R., Bhagi-Damodaran A. Oxygen Sensing, Methods in Molecular Biology. (2023) https://doi.org/10.1007/978-1-0716-3080-8_1