Breaking the strongest bond to carbon
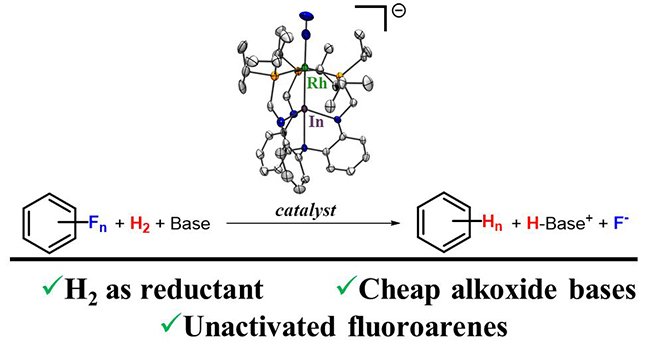
Chemistry researchers James "JT" Moore and Professor Connie Lu have discovered a path for breaking carbon-flouride (C-F) bonds. This work was published in the Journal of the American Chemical Society, "Catalytic Hydrogenolysis of Aryl C–F Bonds Using a Bimetallic Rhodium–Indium Complex."
Organofluorines, organic compounds containing C-F bonds, are almost all man-made chemicals and are widely used as pharmaceuticals, refrigerants, agrochemicals, and surfactants. The C-F bond is one of the strongest in organic chemistry, giving rise to some of the desirable properties of organofluorines. Unfortunately, this same property also renders them nearly indestructible. Organofluorines, in particular perfluoroalkyl substances (PFAs), are now recognized to persist and accumulate in the environment and can be highly toxic to animals and humans.
Moore and Lu discovered that a bimetallic Rh-In catalyst that transforms aryl C-F bonds to C-H bonds using hydrogen gas (H2). The process of removing the F atom in an organofluorine and replacing it with a H atom is called hydrodefluorination. This process is important for treating organofluorines to render them less harmful. Many catalysts are known to hydrodefluorinate organofluorines, but they suffer from two drawbacks: (1) they only remove relatively weak C-F bonds in polyfluorinated arenes, leaving behind mono- or difluoroarenes that are much less reactive; and (2) they use speciality / expensive reagents such as silanes and boranes because the formation of strong Si-F and B-F bonds can help compensate for breaking the strong C-F bond. Moore and Lu's catalyst addresses both of these issues by hydrodefluorinating the difficult mono- and di-fluoroarene subtrates using H2 and a simple alkoxide base. H2 is more sustainable as a reagent than those silanes and boranes.
This discovery was truly accidental. Moore was crystallizing a Rh-In complex in a solvent mixture of fluorobenzene and benzene. The complex cleaved the C-F bond of fluorobenzene to release F- and form a new Rh-C(Ph) bond. The researchers were able to capitalize on this serendipitous reaction to develop our hydrodefluorination process. Moore also isolated the other intermediates in the proposed catalytic cycle.
In the future, the researchers are targeting catalysts to break an even stronger C-F bond, the alkyl C-F bond that are found in PFAs. The PFAs have been in the news lately because of their widespread contamination in drinking waters and are highly toxic to humans.