A trio of papers highlights utility of synthetic probes for exploring protein-lipid modification

MINNEAPOLIS / ST. PAUL (11/9/2023) – The Distefano group in the University of Minnesota Department of Chemistry is making strides in protein-lipid research. Three recent papers weave a compelling narrative, highlighting the pivotal role of chemical probes in advancing our understanding of crucial biological processes.
Lipid-modified proteins play central roles in regulating the flow of information from outside the cell to the inside where patterns of gene transcription change in response to those external stimuli. Chemical probes that allow the levels of many lipid-modified proteins to be measured simultaneously provide important insights into global regulatory circuits controlling cellular behavior. In a paper published in Nature Communications involving graduate student Shelby Auger from the Distefano group – in collaboration with investigators at Harvard Medical School and Moderna – the discovery of a new enzyme that catalyzes isoprenoid group removal was reported. Metabolic labeling using a synthetic analogue of the natural substrate showed the importance of this enzyme in modulating immune response to bacterial infection.
In a second study, described in the Journal of the American Chemical Society, Shelby Auger, Riki Das and Kiall Suazo from the Distefano group, along with collaborators at the National Institutes of Health, used chemical genetics involving lipid probes functionalized with bulky aryl substituents to determine the substrate scope for several lipid-transferring enzymes. Those experiments led to the discovery of a family of lipid-modified proteins that modulate COVID infection.
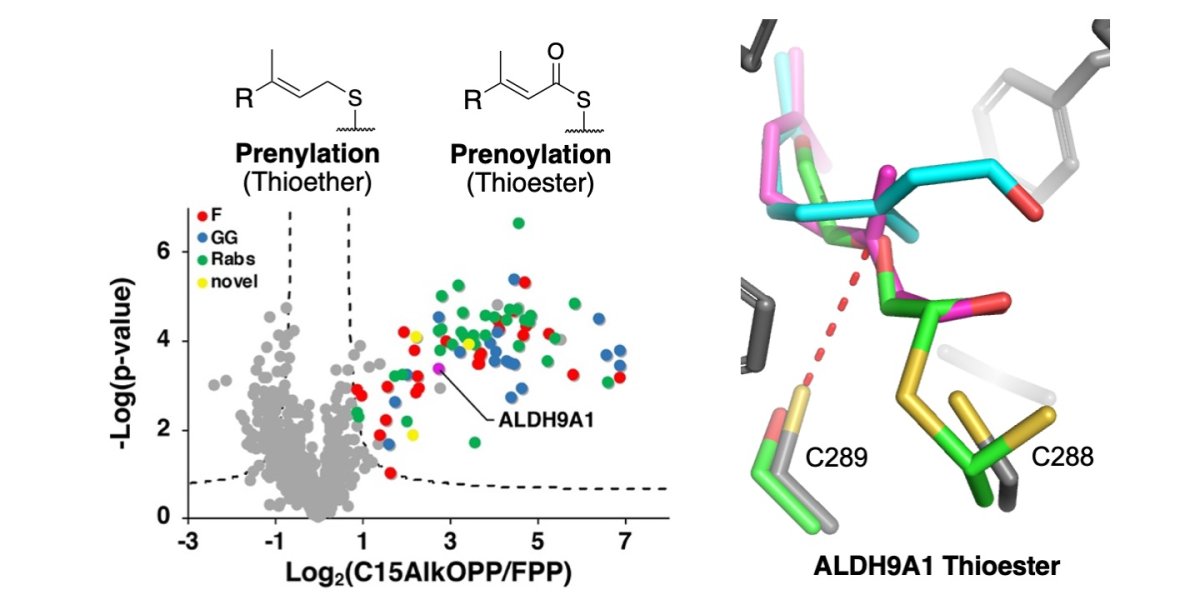
In a third paper, Kiall Suazo, Garrett Schey, Shelby Auger, Alex Petre from the Distefano group – and investigators in Poland and the Czech Republic – reported a new type of protein lipid modification involving thioesterification in RSC Chemical Biology. Collectively, these three examples highlight the utility of synthetic analogues incorporating bio-orthogonal functionality for studying critical biological processes and demonstrate how synthetic chemistry in concert with mass spectrometry allows cellular processes to be interrogated on a global scale.
The Distefano group is made up of eight graduate students and one postdoctoral researcher. Their research is centered on the chemical biology and biotechnology applications of protein prenylation. Prof. Distefano founded the group in 1992 when he joined the department as an Assistant Professor. Over the past 30 years, over 160 scholars – including postdocs, visiting researchers, undergraduate and graduate UMN students, and summer undergraduate researchers – have been involved in the group’s synthetic organic chemistry research.