Past Seminars & Events
Professor Joseph Francisco
Thursday, Sept. 30, 2021, 4 p.m. through Thursday, Sept. 30, 2021, 5 p.m.
This seminar will be presented remotely and live-streamed
Location To Be Determined
Zoom Link
Izaak M. Kolthoff Lectureship in Chemistry
Professor Joseph Francisco
Department of Chemistry and Department of Earth and Environmental Science
University of Pennsylvania
Host: Professor Don Truhlar
A Fresh Look at the Chemistry Behind Acid Rain
The two major components of acid rain are sulfuric acid (H2SO4) and nitric acid (HNO3). Sulfur dioxide (SO2) is the main precursor of H2SO4. Atmospheric sulfur dioxide is oxidized homogeneously by reaction of SO2 with OH and O2 leading to SO3, which then reacts with water to form sulfuric acid. This is the now accepted acid rain mechanism for generation of atmospheric sulfuric acid. In this talk we will review the traditional acid rain mechanism and we will introduce a new acid rain mechanism that relies on the photochemistry of SO2 and show how this new chemistry can be an important ingredient in the overall mechanism of acid rain formation not yet considered by current atmospheric models.
Sulfur dioxide has been proposed in solar geoengineering as a precursor of H2SO4 aerosol, a cooling agent active in the stratosphere to contrast climate change due to the anthropogenic emissions of greenhouse carbon dioxide. Considering the introduction of SO2 in the stratosphere, the photochemistry of HOSO is critical to understanding the role of SO2 mitigating climate change. The spectroscopy and photochemistry this new species provide important insights that help to better understand SO2 chemistry in earth's upper atmosphere.
Research
Professor Joseph S. Francisco’s laboratory focuses on basic studies in spectroscopy, kinetics, and photochemistry of novel transient species in the gas phase. He has made significant contributions in many areas of atmospheric chemistry by applying new tools from experimental physical and theoretical chemistry to atmospheric chemical problems. His research has transformed our understanding of chemical processes in the atmosphere at the molecular level. Francisco’s work has led to important discoveries of new chemistries occurring on the interfaces of cloud surfaces as well as fundamental new types of chemical bonding that control these processes.
Professor Joseph S. Francisco
Francisco received his bachelor’s degree from the University of Texas at Austin in 1977 and his doctorate from Massachusetts Institute of Technology in 1983. From 1983-85, Francisco trained as a Research Fellow at the University of Cambridge in England, and then returned to MIT as a Provost Postdoctoral Fellow. He was also a Visiting Associate in Planetary Science at the California Institute of Technology.
Over his career to date, Francisco has published more than 700 journal articles, written several book chapters, and he is the co-author of the fundamental textbook in chemical kinetics and dynamics, Chemical Kinetics and Dynamics. He is a recipient of the Alexander von Humboldt U.S. Senior Scientist Award, the Edward W. Morley Medal from the Cleveland Section of the American Chemical Society, and a John Simon Guggenheim Fellowship. Francisco is a Fellow of the American Chemical Society, the American Physical Society, the American Association for the Advancement of Science, and the American Academy of Arts and Sciences. He is also a Member of the National Academy of Sciences, the American Philosophical Society, and the German National Academy of Sciences Leopoldina.
Francisco is currently the Executive Editor of the Journal of the American Chemical Society, and he has recently been appointed as a member of the Editorial Board for the Proceedings of the National Academy of Sciences. From 2005-07 he served as President of the National Organization for the Professional Advancement of Black Chemists and Chemical Engineers and was President of the American Chemical Society in 2010. Also in 2010, Francisco was appointed to the President’s Committee on the National Medal of Science by President Barack Obama.
Dr. Susan Reutzel-Edens
Thursday, Sept. 23, 2021, 9:45 a.m. through Thursday, Sept. 23, 2021, 11 a.m.
This seminar will be presented in person and live-streamed
331 Smith Hall
Zoom Link
Margaret C. Etter Memorial Lecture in Materials Chemistry
Dr. Susan Reutzel-Edens
The Cambridge Crystallographic Data Centre
Host: Professor Tom Hoye
Inspiring Medicines Design through Solid State Chemistry
The first step in transforming a molecule to a medicine is invariably identifying a stable crystalline form with which to isolate and purify the drug. Among potentially many different solid form options (polymorphs, hydrates, solvates), the chosen crystalline form will ideally have favourable properties for downstream processing and meet the design requirements of the drug product to ensure consistency in its safety and efficacy profile throughout the shelf life. A molecule’s tendency to exhibit polymorphic behavior is one manifestation of the relative importance of two competing factors - thermodynamics and kinetics - as molecules (or ions) pack in different crystal structures at different rates to minimize their free energy. Experimental solid form screens aim to overcome the kinetic barriers to crystal nucleation and growth en route to producing as many polymorphs as possible, in particular the thermodynamically most stable form.
Crystal structure prediction (CSP) methods have also been developed to address “one of the continuing scandals in the physical sciences” to quote John Maddox in 1988, namely the ability to predict the crystal structure of a compound from its chemical composition. Thanks in part to the CCDC-sponsored blind tests of CSP, which have benchmarked the progress of the algorithms over more than two decades, and the emergence of commercial CSP providers, structure prediction is now widely applied across the pharmaceutical industry.[1] However, the application of ‘in silico’ polymorph screening to pharmaceutical molecules, which have usually undergone thorough experimental screening, has revealed two problems: computational over-prediction and experimental under-estimation.[2] In this presentation, the challenges posed by these problems and the opportunity for data-driven approaches to assess the risk of polymorphism are highlighted. Here we attempt to answer, “What can we learn about how molecules crystallize from the 1.1+ million structures in the Cambridge Structural Database that have crystallized?”
Research
Her research interests include crystal polymorphism, materials design and engineering, crystal nucleation and growth, structure-property relationships, crystal structure prediction and the digital design of drug products.
Dr. Susan Reutzel-Edens
Susan earned her PhD at the University of Minnesota (1991) under the direction of the late Professor Margaret C. Etter, and thereafter joined Eli Lilly and Company. There, she founded the solid form design program and for two decades led a team of cross-functional scientists charged with finding commercially-viable crystalline forms for small-molecule drug products.
She has contributed to the development of more than 150 compounds, is a named inventor on 12 US patents, and has published over 50 papers and book chapters on key aspects of solid form development.
She was elected Fellow of the Royal Society of Chemistry in 2018 and in 2019 joined the Scientific Advisory Board of the Cambridge Crystallographic Data Centre. She is also an adjunct professor at Purdue University and currently serves on the CrystEngComm Editorial Board, the Editorial Advisory Boards of the Journal of Pharmaceutical Sciences and Pharmaceutical Research, and as a topic editor for Crystal Growth and Design.
Professor Valerie Pierre
Thursday, Sept. 16, 2021, 9:45 a.m. through Thursday, Sept. 16, 2021, 11 a.m.
This seminar will be presented in person and live-streamed
331 Smith Hall
Zoom Link
Departmental Seminar
Professor Valerie Pierre
Department of Chemistry
University of Minnesota
Host: Professor Larry Que
Inorganic Receptors for Phosphate for Medical and Environmental Applications
Accumulation of phosphate in many inland and coastal waters primarily due to wastewater discharge and agricultural runoff can lead to eutrophication, causing substantial detrimental environmental impact including harmful algal blooms, fish-kills, and the formation of hypoxic dead zones. Accumulation of phosphate in blood, a condition called hyperphosphatemia, affects most patients with advanced chronic and acute kidney disease and kidney failure. Maintenance hemodialysis does not remove phosphate from blood effectively; thus, almost all patients on maintenance hemodialysis have hyperphosphatemia. The inability to manage this disorder contributes significantly to the increase in morbidity and mortality of advanced kidney disease. Both of these problems could be resolved with immobilized phosphate receptors that have high affinity for phosphate in water and that are highly selective over competing anions, notably bicarbonate and chloride. Through the development of these receptors, we determined how minor differences in the ligand lead to substantial changes in recognition of anions by the metal complex. The nature of the coordinating group, that of the metal ion, the geometry of the metal complex, its charge, and the presence of a hydrogen-bonding network all affect anion recognition, binding, and selectivity. Lastly, in order to be translatable and economically viable, any blood- or water-filtering technology must also be regenerable. This property requires receptors that are both reversible and controllable. The ability of our inorganic receptors complexes to catch phosphate and release it at will upon addition of an external trigger makes them promising candidates for the development of a new class of recyclable selective filtration technology.
Research
The Pierre’s research group exploits coordination and organic chemistry to solve medical and environmental problems. The group uses siderophores, natural products synthesized by bacteria to chelate iron, as a template to design novel chemical probes and imaging agents to rapidly diagnose bacterial infections in vitro and in vivo and to develop antibiotics with improved efficacy against antimicrobial-resistant bacteria.
As part of our environmental efforts, we are designing new complexes, supramolecular receptors and polymeric membranes to remove pollutants and toxic compounds such as phosphates, arsenate and cyanide from surface water
Professor Valerie Pierre
Engineer Diploma 2001 Ecole Superieure de Chimie Lyon, France
Ph.D. 2005 University of California, Berkeley
Post-doctoral Scholar 2005-2007 California Institute of Technology
Professor Edgar Arriaga Kick Off Seminar
Thursday, Sept. 9, 2021, 9:45 a.m. through Thursday, Sept. 9, 2021, 11 a.m.
Departmental Seminar
Professor Edgar Arriaga
Department of Chemistry
University of Minnesota
Host: Professor Valerie Pierre
Expanding the Repertoire for Single Cell Analysis through Mass Cytometry
The analysis of single cells could open the gates to understand the molecular basis of cellular interactions that define the origins of human disease and aging. Early single cell analyses efforts demonstrated feasibility, but lacked the scalability needed in the clinical and biomedical fields. Mass cytometry (a.k.a. Cytometry by Time-of-Flight, CyTOF) is a relatively new technique that has combined flow cytometry and mass spectrometry to define a high-throughput and multi-parametric analysis of single cells. This presentation will describe how we are applying chemical principles to expand the use of mass cytometry, to investigate the foundations of human aging, and expand its scope to the analysis of individual organelles.
Research
Our research efforts combine expertise in bioanalytical chemistry, chemical biology, and biomedical engineering. We develop unique methods and instrumentation to characterize the chemical, biochemical, and physiological properties of single biological cells and their subcellular components (organelles). These resources enable single cell and subcellular studies that are essential to investigate biological complexity, which presently sets limitations to the research carried out in the biomedical and biotechnological fields. Within these fields we strive to help answers key questions related to the aging process, diabetes and obesity and neurodegeneration as we apply our developments to biological models including mammalian cell cultures, murine and human skeletal muscle, and Caenorhabditis elegans.
Professor Edgar Arriaga
B.S. Universidad del Valle de Guatemala, 1985
Ph.D. Dalhousie University in Nova Scotia, 1990
Post-Doctorate University of Kansas Medical Center, 1990-1991, University of Alberta, 1992-1998
3rd-year Graduate Research Symposium
Thursday, May 20, 2021, 10 a.m. through Thursday, May 20, 2021, 3:46 p.m.
Via Zoom
The 20th annual Chemistry Graduate Student Symposium is being held May 20, 2021 virtually on Zoom. The symposium primarily consists of research presentations by third-year graduate students in the Chemistry Ph.D. program at the University of Minnesota. Presentations will take place in four concurrent sessions and will be 20 minutes in length with an additional 5 minutes reserved for discussion
Professor Teri Odom
Thursday, May 6, 2021, 9:45 a.m. through Thursday, May 6, 2021, 11 a.m.
Professor Teri Odom
Department of Chemistry
Northwestern University
Host: Professor Renee Frontiera
Resolving Nano-bio Interactions at the Single-Nanoconstruct Level
Nanotechnology offers unique strategies for minimally invasive and localized approaches to diagnose and treat diseases. For example, nanoparticles have been explored in a range of applications, including as drug delivery vehicles, imaging probes, and therapeutic agents. Although increased therapeutic efficacy has been realized, direct visualization of how engineered nanoparticles interact with specific organelles or cellular components has been limited. Such interactions will have implications for fundamentals in cancer biology as well as in the design of translational therapeutic agents. This talk will describe how drug-loaded gold nanostars can behave as optical probes to interrogate how therapeutic nanoconstructs interact with cells at the nanoscale. We will focus on model cancer cell systems that can be used to visualize how gold nanostar nanoconstructs target cells, rotate and translate on the plasma membrane, are endocytosed, and are trafficked intracellularly. Finally, we will discuss how the different motions provide insight into whether the therapeutic nanoconstructs will retain their targeting abilities.
Professor Odom
Professor Teri Odom's group focuses on “making precious metals more precious” by controlling the size and shape of noble metals at the nanoscale. Her group's strategies include the development of new nanofabrication tools to create three-dimensional architectures with structural function that can span three-orders of magnitude simultaneously. We are also pursuing simple and scalable approaches to synthesize anisotropic particles. To understand the details of how light interacts with these structures, they use modeling to calculate the optical properties of single particles as well as the collective effects of assemblies of nanoparticles. Applications of these unique materials include nanomedicine, photovoltaics, sensing, and imaging.
Professor Odom is the Charles E. and Emma H. Morrison Professor of Chemistry, chair of the Department of Chemistry, and professor of Materials Science and Engineering at Northwestern University. She is editor-in-chief or Nano Letters. She earned her bachelor's degree in chemistry from Stanford University, her doctorate in chemical physics from Harvard University, and was a post-doctoral researcher at Harvard.
Albert J. Moscowitz Memorial Lectureship
The Albert J. Moscowitz Memorial Lectureship in Chemistry was established by friends and colleagues of Professor Albert J. Moscowitz (1929-1996) to honor his many contributions to molecular spectroscopy. He was known for his research on the interpretation of optical rotation and circular dichroism spectra in terms of the structures of chiral molecules. In collaboration with colleagues in the medical sciences, he developed important applications of his methods to biomedical systems. Throughout his career, Moscowitz held numerous visiting professorships at other universities, and served on the editorial boards of the leading journals in chemical physics. His work was honored by election as Foreign Member of the Danish Royal Academy of Sciences and Letters, and as a Fellow of the American Physical Society.
Past Albert J. Moscowitz lecturers include Bruce Berne, Columbia University (2000), R. Stephen Berry, University of Chicago (1998), Jean-Luc Bredas, University of Arizona (2002), Mike Duncan, University of Georgia (2010), Crim F. Fleming, University of Wisconsin (2006), C. Daniel Frisbie, University of Minnesota (1999), Mike Frisch, Gaussian (2008), Anthony Legon, University of Bristol (2013), Marsha Lester, University of Pennsylvania (2011), Frank Neese, Max-Planck Institute for Chemical Energy Conversion (2014), Stuart Rice, University of Chicago (2000), Peter Rossky, University of Chicago (2006), Giacinto Scoles, University of Princeton (2004), Benjamin Schwartz, University of California, Los Angeles (2007), Hirata So, University of Illinois, Urbana-Champaign (2011), Walter Thiel, Max Plank Institute, Muelhiem (2002), Zhen-Gang Wang, CalTech (2014), Georg Kresse, University of Vienna (2016), Emily A. Carter, Princeton University (2017), Martin Moskovits, University of California, Santa Barbara (2018), and Veronique Van Speybroeck, Ghent University (2019).
Professor Ekaterina Pletneva
Thursday, April 29, 2021, 9:45 a.m. through Thursday, April 29, 2021, 11 a.m.
Professor Ekaterina Pletneva
Department of Chemistry
Dartmouth College
Host: Professor Ambika Bhagi-Damodoran
Ligands, Protons, Neighboring Redox Centers, and Protein Fold in Redox Reactions of Heme Proteins
Electron-transfer reactions are essential to function of heme proteins as enzymes and electron carriers. In many of these systems the movement of electrons is coupled to other processes such as changes in protonation and protein conformation. Further, hemes are often incorporated into strings of multiple redox centers and their redox properties are strongly affected by their redox neighbors. Our understanding of these important redox-linked processes is incomplete, in part because they cannot be always readily observed. We employ a number of approaches to probe these elusive phenomena in c-type cytochromes and their relevance to biological redox mechanisms.
A small protein cytochrome c (cyt c) with its flexible coordination loop offers opportunities for engineering differently-ligated heme proteins within the cyt c scaffold. We have engineered a variety of switchable proteins in which the interchanging heme iron ligands are Met, Lys, Cys, and His. Analysis of protein stability demonstrates that the protein scaffold and the polypeptide interactions with the solvent play an important role in stabilizing particular heme coordination. Ligand exchange and accompanying protein rearrangements control the rates of redox reactions in these systems. Variations in the identity and location of the dissociating or incoming ligand alter reaction rates by orders of magnitude. Protonation of the heme iron ligands and neighboring groups modify redox reactivity of our model proteins. We show that enthalpies of protonation from isothermal titration calorimetry can be used to identify the number of involved protons and sites of protonation (deprotonation) in protein redox reactions. Finally, our in vitro and in vivo studies of bacterial electron carriers cyt c4, proteins with two heme groups, illustrate the role of the diheme architecture in tuning the electron injection efficiency of these proteins in their ET reaction with cbb3 oxidases.
Professor Pletneva
Heme proteins are the main subjects of Professor Pletneva's research. In particular, researchers in her group have been focusing on ligand substitution reactions at the heme as a common platform for switching the protein structure and redox reactivity in signaling processes. They are investigating conformational properties of cytochrome c in apoptosis and correlate them to the protein peroxidase activity, which is critical for execution of this cellular pathway. We are also studying redox reactivity and folding of native sensors and engineered "switchable" proteins, in which changes in the oxidation state of the heme are linked to heme ligand substitution resulting in protein conformational rearrangements.
Professor Michael Fayer
Tuesday, April 27, 2021, 10 a.m. through Tuesday, April 27, 2021, 11 a.m.
Professor Michael Fayer
Department of Chemistry
Stanford University
Host: Professor Aaron Massari
Dynamics in concentrated LiCl and HCl solutions
Aqueous salt solutions occur widely in systems ranging from industrial processes to biological materials. Prominent examples include batteries and desalinization. The properties of aqueous electrolyte solutions involve the dynamics of water and the dynamics of ions. A closely relate problem is proton transfer in acid solutions. Proton transfer in water is ubiquitous and a critical elementary event which, via proton hopping between water molecules, enables protons to diffuse much faster than other ions. While there have been a vast number of experiments and molecular dynamics simulations investigating proton hopping in water, a direct experimental observation of proton hopping has remained elusive due to its ultrafast nature and the lack of direct experimental observables. The dynamics of the formation and dissociation of complexes of Li+ and water with methylthiocyanate (MeSCN) in very concentration LiCl solutions are explicated using two dimensional infrared (2D IR) Chemical Exchange Spectroscopy. The CN stretch is used as the vibrational probe. 2D IR spectral diffusion measurement show that MeSCN accurately reports on the hydrogen bond dynamics in pure water, making it an excellent probe of dynamics in aqueous systems. Water forms a hydrogen bond and Li+ associates with the nitrogen lone pair of the CN moiety of MeSCN. These two complexes display distinct CN peaks in the FT-IR spectrum. 2D IR is used to directly measure the chemical exchange of water and Li+ with the nitrogen lone pair of the CN moiety. 2D IR is also used to measure the spectral diffusion, which provides information on the dynamic of the concentrated salt solutions. In pure water, the spectral diffusion gives rise to a biexponential decay of the 2D IR data. In the salt solutions, triexponentials are observe. The slowest component is assigned to the time for ion clusters to randomize. 2D IR Chemical Exchange Spectroscopy was also used to extract the chemical exchange rates between hydronium and water in HCl solutions using MeSCN. Ab initio molecular dynamics simulations demonstrate that the chemical exchange is dominated by proton hopping. The observed experimental and simulated acid concentration dependences as well as a number of factors obtained from the simulations and spectral diffusion measurements make it possible to extrapolate the measured single step proton hopping time in concentrated HCl to the dilute limit. Within error the 2D IR measure hopping time yields the same value as inferred from measurements of the proton diffusion constant. It is found that the dilute limit, the proton hopping time is the same as the time for concerted H-bond rearrangement of the extended H-bond network in pure water. The results indicate that the H-bond rearrangement of the water network in which hydronium ions are embedded triggers proton hopping.
Professor Fayer
Professor Fayer earned his bachelor and master's degrees from the University of California, Berkeley. He was a professor of physics at the University of Grenoble, before joining the faculty at Stanford University. He is the David Mulvane Ehrsam and Edward Curtis Franklin professor of chemistry at Stanford University.
Researchers in Professor Fayer's lab are using ultrafast 2D IR vibrational echo spectroscopy and other multi-dimensional IR methods, which they have pioneered, to study dynamics of molecular complexes, water confined on nm lengths scales with a variety of topographies, molecules bound to surfaces, ionic liquids, and materials such as metal organic frameworks and porous silica. They are also studying dynamics in complex liquids, in particular room temperature ionic liquids, liquid crystals, supercooled liquids as well as in influence of small quantities of water on liquid dynamics. In addition, Professor Fayer is studying photo-induced proton transfer in nanoscopic water environments such as polyelectrolyte fuel cell membranes, using ultrafast UV/Vis fluorescence and multidimensional IR measurements to understand the proton transfer and other processes and how they are influenced by nanoscopic confinement.
Bryce L. Crawford Jr.
Bryce L. Crawford Jr. was a renowned Department of Chemistry professor and scientist. He died in September 2011, at the age of 96. He joined the department in 1940, and became a full professor of physical chemistry in 1946. He was chair of the department from 1955 to 1960, and was dean of the graduate school from 1960 to 1972. He retired in 1985. He loved studying molecular vibrations and force constants, and the experimental side of molecular spectroscopy and molecular structure. During World War II, Crawford worked in research on rocket propellants, making significant contributions to rocketry, and the development of solid propellants for the much larger rockets that evolved after the war. Crawford received many honors during his career, including the prestigious American Chemical Society Priestley Medal; and being named a Fellow of the Society for Applied Spectroscopy, a Guggenheim Fellow at the California Institute of Technology, and a Fulbright Fellow at Oxford University. He held the distinction of membership in three honorary science academies, and was actively involved in many professional associations.
Professor Omar Yaghi
Thursday, April 22, 2021, 9:45 a.m. through Thursday, April 22, 2021, 11 a.m.
Kolthoff Lecture #3
Professor Omar Yaghi
Department of Chemistry
University of California, Berkeley
Host: Professor Theresa Reineke
Water Harvesting from Air Anytime Anywhere
Water is essential to life. It is estimated that by 2050 nearly half of the world population will live in water stressed regions, due to either arid conditions or lack of access to clean water. This presentation outlines the parameters of this vexing societal problem and presents a solution to the global water challenge. Metal−organic frameworks (MOFs) have emerged as a unique class of porous materials capable of trapping water at relative humidity levels as low as 10%, and doing so with facile uptake and release kinetics. From laboratory testing to field trials in the driest deserts, kilogram quantities of MOFs have been tested in several generations of devices. We show that the vision of having clean water from air anywhere in the world at any time of the year is potentially realizable with MOFs and so is the idea of giving “water independence” to the citizens of the world.
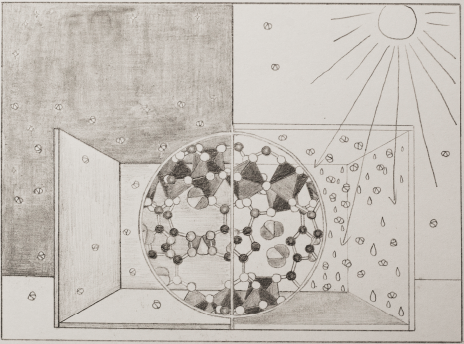
Research
Professor Omar Yaghi's work encompasses the synthesis, structure and properties of inorganic and organic compounds and the design and construction of new crystalline materials. He is widely known for pioneering several extensive classes of new materials termed metal-organic frameworks, covalent organic frameworks, and zeolitic imidazolate frameworks. These materials have the highest surface areas known to date, making them useful in clean energy storage and generation. Specifically, applications of his materials are found in the storage and separation of hydrogen, methane, and carbon dioxide, and in clean water production and delivery, supercapacitor devices, proton and electron conductive systems. The building block approach he developed has led to an exponential growth in the creation of new materials having a diversity and multiplicity previously unknown in chemistry. He termed this field 'Reticular Chemistry' and defines it as stitching molecular building blocks into extended structures by strong bonds.
Professor Yaghi
Professor Yaghi received his Bachelor of Science degree from State University of New York-Albany, and doctorate from the University of Illinois-Urbana. He was a National Science Foundation Post-doctoral fellow at Harvard University. He has been on the faculties of Arizona State University, University of Michigan, and UCLA. He is currently the James and Neeltje Tretter Chair Professor of Chemistry at the University of California, Berkeley, and a senior faculty scientist at Lawrence Berkeley National Laboratory. He is the founding director of the Berkeley Global Science Institute. He is also co-director of the Kavli Energy NanoScience Institute, and the California Research Alliance by BASF.
Kolthoff Lectureship in Chemistry
Izaak Maurits Kolthoff was born on February 11, 1894, in Almelo, Holland. He died on March 4, 1993, in St. Paul, Minnesota. In 1911, he entered the University of Utrecht, Holland. He published his first paper on acid titrations in 1915. On the basis of his world-renowned reputation, he was invited to join the faculty of the University of Minnesota’s Department of Chemistry in 1927. By the time of his retirement from the University in 1962, he had published approximately 800 papers. He continued to publish approximately 150 more papers until his health failed. His research, covering approximately a dozen areas of chemistry, was recognized by many medals and memberships in learned societies throughout the world, including the National Academy of Sciences and the Nichols Medal of the American Chemical Society. Best known to the general public is his work on synthetic rubber. During World War II, the government established a comprehensive research program at major industrial companies and several universities, including Minnesota. Kolthoff quickly assembled a large research group and made major contributions to the program. Many of Kolthoff’s graduate students went on to successful careers in industry and academic life and, in turn, trained many more. In 1982, it was estimated that approximately 1,100 Ph.D. holders could trace their scientific roots to Kolthoff. When the American Chemical Society inaugurated an award for excellence in 1983, he was the first recipient.
Professor Omar Yaghi
Tuesday, April 20, 2021, 9:45 a.m. through Tuesday, April 20, 2021, 11 a.m.
Kolthoff Lecture #2
Professor Omar Yaghi
Department of Chemistry
University of California, Berkeley
Host: Professor Theresa Reineke
The Discovery of Covalent Organic Frameworks
The synthesis of covalently-linked organic extended structures has been a long-standing objective. The fundamental problem is that attempts to link organic molecular building blocks into extended structures often led to intractable amorphous solids and ill-defined materials, thus impeding development of this field. This changed when the reaction and crystallization conditions for making covalent organic frameworks (COFs) were worked out and reported in 2005 for 2D COFs and 2007 for 3D COFs. This advance extended the field of organic chemistry beyond discrete molecules (0D) and polymers (1D) to infinite layered (2D) and network (3D) extended structures. The discovery of reactions and crystallization conditions for making COFs using reversible as well as what is traditionally considered irreversible linkages (e.g. dioxin, olefin) will be outlined. The recent developments in (1) making large single crystals of COFs, (2) the first molecular weavings, and (3) greatly expanding structural complexity of COFs through building high valency nodes will be presented.
Research
Professor Omar Yaghi's work encompasses the synthesis, structure and properties of inorganic and organic compounds and the design and construction of new crystalline materials. He is widely known for pioneering several extensive classes of new materials termed metal-organic frameworks, covalent organic frameworks, and zeolitic imidazolate frameworks. These materials have the highest surface areas known to date, making them useful in clean energy storage and generation. Specifically, applications of his materials are found in the storage and separation of hydrogen, methane, and carbon dioxide, and in clean water production and delivery, supercapacitor devices, proton and electron conductive systems. The building block approach he developed has led to an exponential growth in the creation of new materials having a diversity and multiplicity previously unknown in chemistry. He termed this field 'Reticular Chemistry' and defines it as stitching molecular building blocks into extended structures by strong bonds.
Professor Yaghi
Professor Yaghi received his Bachelor of Science degree from State University of New York-Albany, and doctorate from the University of Illinois-Urbana. He was a National Science Foundation Post-doctoral fellow at Harvard University. He has been on the faculties of Arizona State University, University of Michigan, and UCLA. He is currently the James and Neeltje Tretter Chair Professor of Chemistry at the University of California, Berkeley, and a senior faculty scientist at Lawrence Berkeley National Laboratory. He is the founding director of the Berkeley Global Science Institute. He is also co-director of the Kavli Energy NanoScience Institute, and the California Research Alliance by BASF.
Kolthoff Lectureship in Chemistry
Izaak Maurits Kolthoff was born on February 11, 1894, in Almelo, Holland. He died on March 4, 1993, in St. Paul, Minnesota. In 1911, he entered the University of Utrecht, Holland. He published his first paper on acid titrations in 1915. On the basis of his world-renowned reputation, he was invited to join the faculty of the University of Minnesota’s Department of Chemistry in 1927. By the time of his retirement from the University in 1962, he had published approximately 800 papers. He continued to publish approximately 150 more papers until his health failed. His research, covering approximately a dozen areas of chemistry, was recognized by many medals and memberships in learned societies throughout the world, including the National Academy of Sciences and the Nichols Medal of the American Chemical Society. Best known to the general public is his work on synthetic rubber. During World War II, the government established a comprehensive research program at major industrial companies and several universities, including Minnesota. Kolthoff quickly assembled a large research group and made major contributions to the program. Many of Kolthoff’s graduate students went on to successful careers in industry and academic life and, in turn, trained many more. In 1982, it was estimated that approximately 1,100 Ph.D. holders could trace their scientific roots to Kolthoff. When the American Chemical Society inaugurated an award for excellence in 1983, he was the first recipient.