Tinnitus treatment device featured in Nature Communications
September 11, 2024 — A research paper highlighting the clinical trial results of a unique tinnitus therapy device, Lenire, was recently published in Nature Communications and selected as the featured article on its website.
Lenire was developed by Neuromod Devices, a global medical technology company founded to establish bimodal neuromodulation as a new standard of care for tinnitus. Department of Biomedical Engineering Professor Hubert Lim is Chief Scientific Officer of the company and the corresponding author of the Nature Communications article, “Combining sound with tongue stimulation for the treatment of tinnitus: a multi-site single-arm controlled pivotal trial.”
Treating tinnitus through bimodal neuromodulation
Tinnitus is perceived as ringing in the ears when no sound is actually present. It is a common condition that affects 10 to 15 percent of the population. For some, the condition can interfere with concentration and hearing, greatly affecting quality of life. Until recently, tinnitus had few effective treatment options.
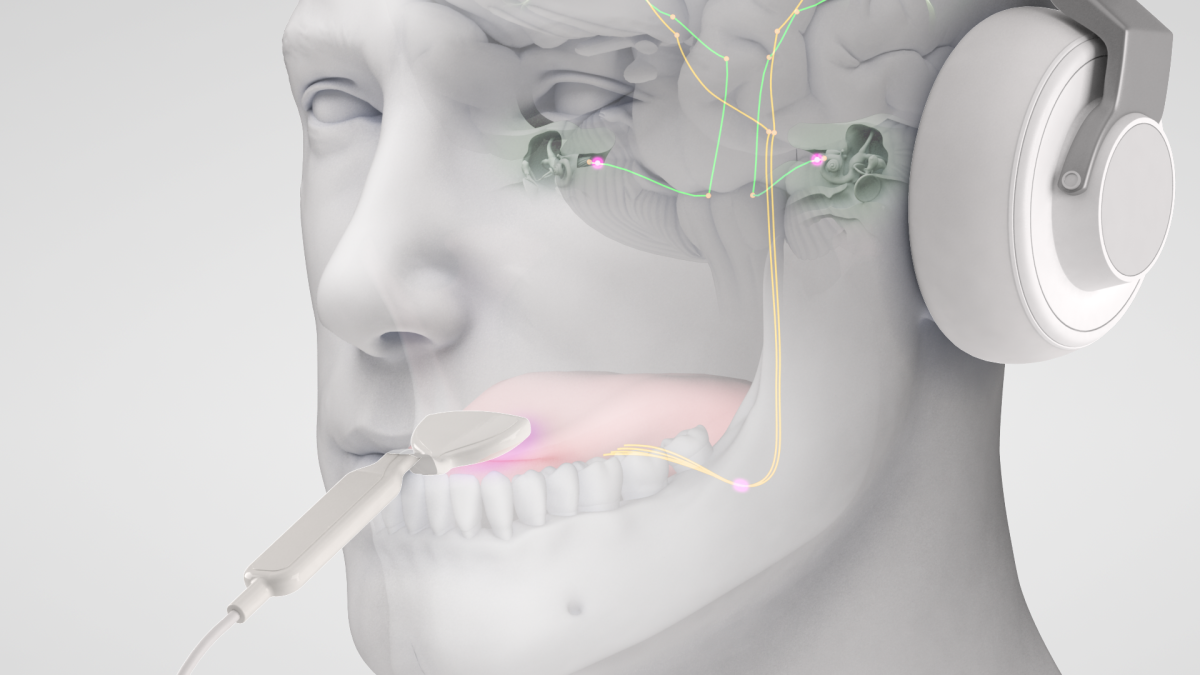
The Lenire device was designed to treat tinnitus through bimodal neuromodulation with sounds presented to the ears and gentle electrical pulses to the tongue surface. The device plays specific sounds through earphones to condition the brain to become less and less focused on the tinnitus. Simultaneously, the device electrically stimulates the tongue to reinforce the effect.
The clinical study was designed to compare the effect of sound-only treatment to bimodal treatment on tinnitus sufferers. The study followed 112 participants through 12 weeks of treatment: 6 weeks with sound-only treatment and 6 weeks with bimodal treatment that included tongue stimulation.
Controlled pivotal trial demonstrates efficacy and safety
The study found that 58.6 percent of moderate or more severe tinnitus sufferers responded to bimodal treatment compared to only 43.2 percent using sound-only treatment, demonstrating the superiority of bimodal neuromodulation in treating more severe cases of tinnitus.
The research also revealed that 79.4 percent of participants experienced clinically meaningful improvement in the tinnitus over the entire 12 weeks of the study.
Importantly, the trial revealed no severe adverse effects associated with use of the Lenire device, and the FDA granted de novo approval of the device based on this research and the therapeutic effects of the device on 204 real-world patients.
Lenire has been available to tinnitus specialists since March 2023, and a manuscript outlining real-world device performance was recently submitted to a journal for review. The real-world performance has shown encouraging outcomes in 212 patients treated with Lenire that surpassed the positive effects observed in the clinical trial.
Additional paper authors include Michael Boedts, Andreas Buechner, S. Guan Khoo, Welmoed Gjaltema, Frederique Moreels, Anke Lesinski-Schiedat, Philipp Becker, Helen MacMahon, Lieke Vixseboxse, Razieh Taghavi, and Thomas Lenarz.