Multiple bonds at UMn in uranium–transition metal complexes
Metal–metal bonding is an important concept that helps rationalizing the structure, reactivity, metal–surface chemistry, and catalysis of metal complexes. Compared to transition metals, the nature of metal–metal bonds involving the d–f heterobimetallic bonding has been less explored.
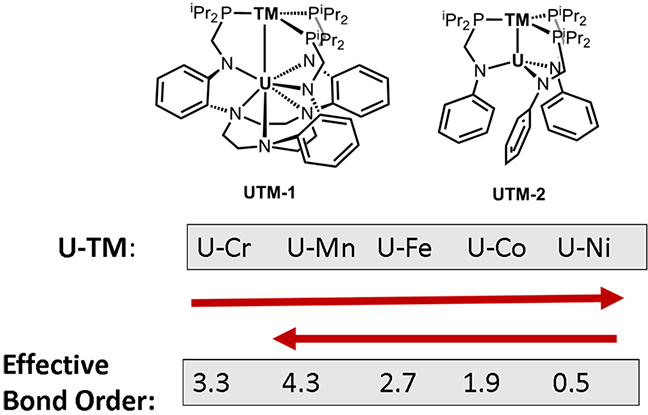
In a study carried out by graduate student Prachi Sharma working with Professor Laura Gagliardi, in collaboration with graduate student Bianca L. Ramirez working with Professor Connie Lu, two new heterobimetallic complexes containing uranium and first-row transition metal have been predicted using quantum chemical methods.
The systematic study of the interaction between uranium and a first-row transition metal demonstrates the wide tunability of bond orders across the period, ranging from formally a quintuple to a single bond according to the nature of the transition metal. The calculations predict a 5-fold bonding between uranium and manganese in the UMn(iPr2PCH2NPh)3 complex, which is unprecedented in the literature.
This work has been published in Inorganic Chemistry. It follows a previous experimental and computational study of the two groups in which the same ligands were employed to make 4f-3d Lu-Ni bimetallic species.