Past Seminars & Events
Professor John Anderson
Tuesday, March 16, 2021, 9:45 a.m. through Tuesday, March 16, 2021, 11 a.m.
Professor John Anderson
Department of Chemistry
University of Chicago
Host: Professor Ian Tonks
Synthesis and Reactivity of a Terminal Co Oxo Complex
Transition metal oxo complexes, particularly those of later transition metals, have been cited as important intermediates in processes such as C-H hydroxylation and oxygen evolution. In general, however, these species are highly reactive and difficult to isolate and study. In this talk I will present our laboratory’s efforts at isolating and studying late transition metal oxo complexes. We have found that use of pseudo-tetrahedral geometries enables the isolation of an unusual terminal Co-oxo complex. The reactivity of this species towards C-H activation is markedly different than that observed for the majority of other systems and provides experimental evidence for a new mechanistic scenario dubbed "asynchronous" CPET. Finally, the possible relevance of related Co and Ni complexes in this system towards O-O bond formation will be discussed.
Professor Anderson
John was born in Downers Grove, a suburb about 40 minutes west of Chicago. He acquired his interest in chemistry at an early age from his grandmother, who was a chemist at Abbott Laboratories in North Chicago. Seeking to further his study of science, John matriculated at the University of Chicago. Once there, John quickly joined the laboratory of Professor Greg Hillhouse. It was in the three years that John spent in Greg's lab that he found his love for inorganic chemistry. During this time, John focused on researching phosphine complexes of Ni, specifically with respect to their reactivity with small molecules such as carbon dioxide and carbon disulfide.
After graduating John began his graduate studies in Boston at MIT in the group of Professor Jonas Peters. His time in Boston was to be short-lived however, as the Peters group moved to the California Institute of Technology in sunny Pasadena, CA quickly thereafter. John also made the move and received his Ph.D. from Caltech. John's thesis centered around a discrete Fe complex that mediates catalytic nitrogen fixation to ammonia.
After finishing his thesis, John came to Northwestern to work in the laboratory of Dave Harris. During this time, John has focused on materials, particularly metal organic frameworks. A central theme is the ability to stabilize reactive species, such as low coordinate dioxygen adducts, within metal organic frameworks thus allowing their characterization and study.
In his independent career, John and the Anderson group have been interested in linking the physical properties of transition metal centers, particularly their spin and radical character, to reactivity and bulk properties.
Professor Lyudmila Slipchenko
Thursday, Feb. 25, 2021, 9:45 a.m. through Thursday, Feb. 25, 2021, 11 a.m.
Professor Lyudmila Slipchenko
Department of Chemistry
Purdue University
Host: Professor Jason Goodpaster
Understanding energy transfer in photosynthesis from polarizable models
Solar energy capture and conversion in plants and bacteria is governed by structure and interactions in photosynthetic pigment-protein complexes. In principle, theory and modeling can provide detailed picture of atomistic interactions in photosynthetic complexes. However, large size of molecular systems and complexity of underlying phenomena make practical simulations challenging, such that their accuracy is still far from having predictive power. Here, by rigorous comparison to experimental data, we show that describing protein environment with the polarizable effective fragment potential (EFP) method provides realistic description of excitonic interactions in one of the extensively studied photosynthetic systems, the Fenna-Matthews-Olson (FMO) pigment-protein complex. We decompose protein effects on electronic excitation energies into polarization and electrostatics components of individual amino acids. We also decipher specific mechanisms of how excitonic interactions in FMO are affected by local mutations. Thus, our simulations provide structure-function relations for excitonic interactions and energy transfer in FMO, and pave the way for predictive modeling of electronic properties and spectroscopy in other photosynthetic complexes.
Professor Slipchenko
The focus of Professor Slipchenko's research is on the development of theoretical and computational approaches targeting the electronic structure of extended systems such as photosynthetic and fluorescent proteins, molecular solids, polymers, and bulk liquids. Specifically, researchers develop universal force fields, QM/MM (quantum mechanics/molecular mechanics), and fragmentation techniques. These methods are broadly applicable to all areas of science and engineering; the resulting computer codes are implemented in the Q-Chem and GAMESS electronic structure packages. They also use the developed techniques to investigate fundamental aspects of non-covalent interactions and the effect of the environment on electronic structure.
Professor Eric Schelter
Tuesday, Feb. 23, 2021, 9:45 a.m. through Tuesday, Feb. 23, 2021, 11 a.m.
Professor Eric Schelter
Department of Chemistry
University of Pennsylvania
Host: Valérie Pierre
Fundamental Principles in Coordination Chemistry Applied to Metal Ion Separations for Outcomes in Sustainability
Metals such as gold, palladium, tellurium, lithium, and the rare earths are now pervasive in technology and used regularly in our daily lives. But where do they come from, and how do we get them into pure forms for use in technology? In many cases, mining and purification practices for ‘critical’ metals and extremely harmful for people and the environment. It is therefore attractive to try and reclaim such metals from spent technologies. However, in many cases, chemistry and engineering to recycle specific critical metals is lacking, compared to the cost of obtaining them from primary sources. In this talk, efforts to develop new separations chemistry for recycling critical metals will be presented. Among these, efficient, inter-f-element separations, such as within the rare earths, remain a perennial challenge. We have been interested in triggering element-specific changes, for example through highly specific structural differences, to achieve efficient separations through new thermodynamic modes. And in an orthogonal approach, to express differences in metal complexes through variable rates of some chemical change – a separations chemistry through kinetics. Both methods allow direct connection of coordination chemistry to macroscopic properties for separations. These connections have enabled new modes in solid-liquid extraction to complement solvent extraction for specialized applications. For this talk, our latest results on chelating and redox active ligand frameworks and their applications in thermodynamic and kinetic separations of elements will be presented.
Professor Schelter
Research projects in Professor Schelter’s group involve inert atmosphere/Schlenk line synthesis of inorganic and organometallic complexes. Rigorous characterization of new compounds is achieved through X-ray crystallography, NMR, FTIR, and UV-Visible absorption spectroscopies, electrochemistry and magnetic susceptibility studies. Current projects are focused on the chemistries and electronic structure effects of the lanthanides, uranium and main group elements.
Professor Schelter joined the faculty at the University of Pennsylvania in 2009. He earned his bachelor’s degree from Michigan Technology University, and his doctorate from Texas A&M University. He was a post-doctoral fellow at the Los Alamos National Laboratory.
Professor Lasse Jensen
Thursday, Feb. 18, 2021, 9:45 a.m. through Thursday, Feb. 18, 2021, 11 a.m.
Professor Lasse Jensen
Department of Chemistry
Pennsylvania State University
Host: Professor Renee Frontiera
Surface-Enhanced Spectroscopy in Inhomogeneous Electric Fields
Over the last few years, we have developed new theories and computational methods for understanding vibrational spectroscopy of molecules near metal surfaces. Specifically, we have developed a new computational toolbox for simulating surface-enhanced vibrational spectroscopy in inhomogeneous electric field. This kind of spectroscopy relies on the strong localized electric near-field at the surface of plasmonic metal nanoparticles. Our work has shown that it is possible to resolve intricate molecule vibrations with atomic resolution, which requires that the near-field is confined to a few Ångstroms. Under these conditions, the traditional selection rules breaks down and simulations are required for understanding the spectroscopy. Here, we will discuss our latest developments in understanding surface-enhanced vibrational spectroscopy in inhomogeneous electric fields.
Professor Jensen
Professor Jensen's research lies in the field of theoretical chemistry and involves developing new methods for simulations of metal-molecule interactions. Researchers in his group seek to use computer simulations to gain a fundamental understanding of the underlying physics and chemistry. They are particularly interested in understanding the optical properties of molecules at the interface of plasmonic nanomaterials. The theoretical and computational methods developed and applied in our group combine electronic structure theory and electrodynamics to describe light-matter interactions at the nanoscale.
Professor Timothy Swager
Thursday, Feb. 11, 2021, 9:45 a.m. through Thursday, Feb. 11, 2021, 11 a.m.
Kolthoff Lecture #3
Professor Timothy Swager
Department of Chemistry
Massachusetts Institute of Technology
Host: Professor Marc Hillmyer
Chemistry of Dynamic Liquid-Liquid Interfaces
This lecture will focus on the design of systems wherein reconfiguration of complex liquid emulsions (droplets) can be triggered chemically or biochemically. The utility of these methods is to generate new transduction mechanisms by which chemical and biological sensors can be developed. Complex liquid droplets behave as optical lens systems and small changes in surface tensions can change focal lengths or cause systems to switch between optically transmissive or scattering states. Central to this scheme is that the fluids in the droplets have different densities and hence are aligned by the earth’s gravity. The induced optical changes can be triggered with chemical, photochemical, or biochemical stimuli and thereby create new generations of sensors. Demonstrations of these methods for the detection of enzyme concentrations, pathogens, and antibodies will be presented. Droplets containing birefringent liquid crystals (LCs), including chiral nematic phases, have been prepared and designer surfactants cause either planar of vertical anchoring at the water-liquid crystal interface. The liquid crystals can be used for precise positioning of magnetic particles and biomolecular elements. Magnetic particles can be used to create novel optical functions, including steering of light and selective reflection, which will be detailed.
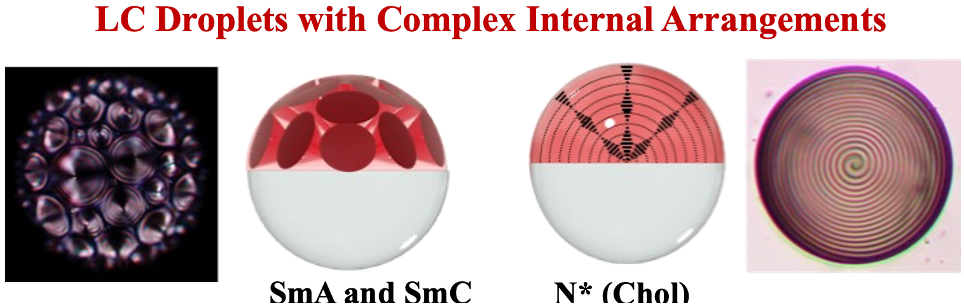
Research
Professor Timothy Swager's research is at the interface of chemistry and materials science, with specific interests in carbon nanomaterials, polymers, and liquid crystals. Reseachers in his group are interested in a spectrum of topics with an emphasis on the synthesis and construction of functional assemblies. Molecular recognition pervades a great deal of their research. Chemosensors require recognition elements to discriminate chemical signals. Electronic polymers are one of the areas that the Swager group is well known for having made many innovations. They are constantly developing new electronic structures, properties, and uses for these materials. Recently, Swager's group launched an effort to create functionalized carbon nanotubes and graphenes. Researchers have advanced new chemical methods for their functionalization and utilization in electrocatalysis and chemical and radiation sensing. In the area of liquid crystals, they make use of molecular complimentarity and receptor-ligand interactions to provide novel organizations.
Professor Swager
Professor Swager joined the Department of Chemistry at Massachusetts Institute of Technology in 1996, holding the title of John D. MacArthur Professor of Chemistry. He is also director of the Deshpande Center for Technological Innovation. He is a member of the National Academy of Sciences and American Academy of Arts and Sciences. He earned his Bachelor of Science in chemistry from Montana State University, and his doctorate at the California Institute of Technology. He was a post-doctoral fellow at MIT. Before joining the faculty at MIT, he was a professor at the University of Pennsylvania.
Kolthoff Lectureship in Chemistry
Izaak Maurits Kolthoff was born on February 11, 1894, in Almelo, Holland. He died on March 4, 1993, in St. Paul, Minnesota. In 1911, he entered the University of Utrecht, Holland. He published his first paper on acid titrations in 1915. On the basis of his world-renowned reputation, he was invited to join the faculty of the University of Minnesota’s Department of Chemistry in 1927. By the time of his retirement from the University in 1962, he had published approximately 800 papers. He continued to publish approximately 150 more papers until his health failed. His research, covering approximately a dozen areas of chemistry, was recognized by many medals and memberships in learned societies throughout the world, including the National Academy of Sciences and the Nichols Medal of the American Chemical Society. Best known to the general public is his work on synthetic rubber. During World War II, the government established a comprehensive research program at major industrial companies and several universities, including Minnesota. Kolthoff quickly assembled a large research group and made major contributions to the program. Many of Kolthoff’s graduate students went on to successful careers in industry and academic life and, in turn, trained many more. In 1982, it was estimated that approximately 1,100 Ph.D. holders could trace their scientific roots to Kolthoff. When the American Chemical Society inaugurated an award for excellence in 1983, he was the first recipient.
Professor Timothy Swager
Wednesday, Feb. 10, 2021, 4 p.m. through Wednesday, Feb. 10, 2021, 5 p.m.
Kolthoff Lecture #2
Professor Timothy Swager
Department of Chemistry
Massachusetts Institute of Technology
Host: Professor Marc Hillmyer
Translating Chemical Reactions and Catalysis to Nano-Electronic Sensors
This lecture will detail the creation of ultrasensitive sensors based on chemiresistive mechanisms with carbon nanotubes (CNTs). A central concept that a single nano- or molecular-wire spanning between two electrodes would create an exceptional sensor if binding of a molecule of interest to it would block all electronic transport. Nanowire networks of CNTs provide for a practical approximation to the single nanowire scheme. These methods include abrasion deposition and selectivity is generated by covalent and/or non-covalent binding selectors/receptors to the carbon nanotubes. Sensors for a variety of materials and cross-reactive sensor arrays will be described. A current limitation to most, it not all sensors, is chemical selectivity. Synthetic receptors can give some selectivity and when they have 3D structures can be highly specific for recognition of a molecule. However, translating complex molecular constructions into strong readable sensory signals is challenging. I will give multiple examples of how established chemical reactions that occur in solution can be used to create highly specific and sensitive sensors. There is a vast opportunity in translating the products of synthetic and catalytic chemistry into selective chemiresistive sensors. I will highlight the utility of CNT-based gas sensors for the detection of alkenes and other gases relevant to agricultural and food production/storage/transportation are being specifically targeted and can be used to create systems that increase production, manage inventories, and minimize losses.

Research
Professor Timothy Swager's research is at the interface of chemistry and materials science, with specific interests in carbon nanomaterials, polymers, and liquid crystals. Reseachers in his group are interested in a spectrum of topics with an emphasis on the synthesis and construction of functional assemblies. Molecular recognition pervades a great deal of their research. Chemosensors require recognition elements to discriminate chemical signals. Electronic polymers are one of the areas that the Swager group is well known for having made many innovations. They are constantly developing new electronic structures, properties, and uses for these materials. Recently, Swager's group launched an effort to create functionalized carbon nanotubes and graphenes. Researchers have advanced new chemical methods for their functionalization and utilization in electrocatalysis and chemical and radiation sensing. In the area of liquid crystals, they make use of molecular complimentarity and receptor-ligand interactions to provide novel organizations.
Professor Swager
Professor Swager joined the Department of Chemistry at Massachusetts Institute of Technology in 1996, holding the title of John D. MacArthur Professor of Chemistry. He is also director of the Deshpande Center for Technological Innovation. He is a member of the National Academy of Sciences and American Academy of Arts and Sciences. He earned his Bachelor of Science in chemistry from Montana State University, and his doctorate at the California Institute of Technology. He was a post-doctoral fellow at MIT. Before joining the faculty at MIT, he was a professor at the University of Pennsylvania.
Kolthoff Lectureship in Chemistry
Izaak Maurits Kolthoff was born on February 11, 1894, in Almelo, Holland. He died on March 4, 1993, in St. Paul, Minnesota. In 1911, he entered the University of Utrecht, Holland. He published his first paper on acid titrations in 1915. On the basis of his world-renowned reputation, he was invited to join the faculty of the University of Minnesota’s Department of Chemistry in 1927. By the time of his retirement from the University in 1962, he had published approximately 800 papers. He continued to publish approximately 150 more papers until his health failed. His research, covering approximately a dozen areas of chemistry, was recognized by many medals and memberships in learned societies throughout the world, including the National Academy of Sciences and the Nichols Medal of the American Chemical Society. Best known to the general public is his work on synthetic rubber. During World War II, the government established a comprehensive research program at major industrial companies and several universities, including Minnesota. Kolthoff quickly assembled a large research group and made major contributions to the program. Many of Kolthoff’s graduate students went on to successful careers in industry and academic life and, in turn, trained many more. In 1982, it was estimated that approximately 1,100 Ph.D. holders could trace their scientific roots to Kolthoff. When the American Chemical Society inaugurated an award for excellence in 1983, he was the first recipient.
Professor Timothy Swager
Tuesday, Feb. 9, 2021, 9:45 a.m. through Tuesday, Feb. 9, 2021, 11 a.m.
Kolthoff Lecture #1
Professor Timothy Swager
Department of Chemistry
Massachusetts Institute of Technology
Host: Professor Marc Hillmyer
Functional Materials from Bicyclic Bridged Building Blocks
The ability of triptycene and related structures to produce materials with unusual properties is simply remarkable. Beyond their initial utility in preventing self-quenching in emissive semiconducting polymers for chemical sensors, we have found that they can guide and enhance alignment to liquid crystals, produce high modulus low dielectric constant materials, functional as gas permeable materials, simultaneously give dramatic increases in strength and ductility of polymers, and provide for novel electronic interactions. In this lecture, I will detail our recent triptycene polymer efforts including: (1) post-polymerization functionalization to give materials with high proton and anion conductivities; (2) scalable mechanochemical synthesis of materials that behave as chemical sponges for aromatic molecules; (3) polymerization of shape persistent iptycene macromonomers to produce high free volume materials; (4) the use of high free volume to create high performance gas separation membranes; (5) iptycene materials as stabilizing hosts for catalytic nanoparticles; and (6) polymers and molecules with electronically active elements that communicate by homo-conjugation to give thermally activated delayed fluorescence.
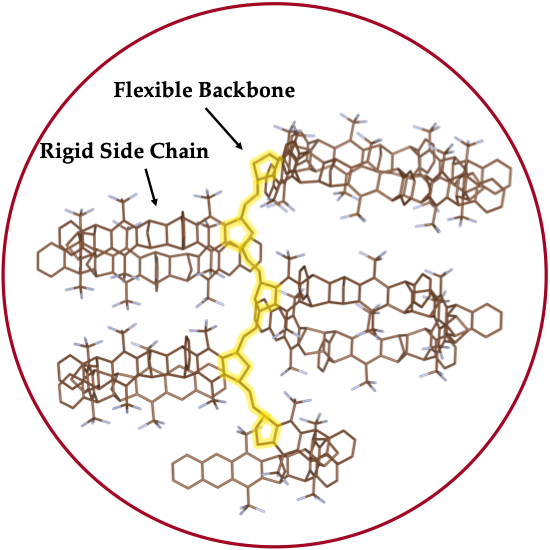
Research
Professor Timothy Swager's research is at the interface of chemistry and materials science, with specific interests in carbon nanomaterials, polymers, and liquid crystals. Reseachers in his group are interested in a spectrum of topics with an emphasis on the synthesis and construction of functional assemblies. Molecular recognition pervades a great deal of their research. Chemosensors require recognition elements to discriminate chemical signals. Electronic polymers are one of the areas that the Swager group is well known for having made many innovations. They are constantly developing new electronic structures, properties, and uses for these materials. Recently, Swager's group launched an effort to create functionalized carbon nanotubes and graphenes. Researchers have advanced new chemical methods for their functionalization and utilization in electrocatalysis and chemical and radiation sensing. In the area of liquid crystals, they make use of molecular complimentarity and receptor-ligand interactions to provide novel organizations.
Professor Swager
Professor Swager joined the Department of Chemistry at Massachusetts Institute of Technology in 1996, holding the title of John D. MacArthur Professor of Chemistry. He is also director of the Deshpande Center for Technological Innovation. He is a member of the National Academy of Sciences and American Academy of Arts and Sciences. He earned his Bachelor of Science in chemistry from Montana State University, and his doctorate at the California Institute of Technology. He was a post-doctoral fellow at MIT. Before joining the faculty at MIT, he was a professor at the University of Pennsylvania.
Kolthoff Lectureship in Chemistry
Izaak Maurits Kolthoff was born on February 11, 1894, in Almelo, Holland. He died on March 4, 1993, in St. Paul, Minnesota. In 1911, he entered the University of Utrecht, Holland. He published his first paper on acid titrations in 1915. On the basis of his world-renowned reputation, he was invited to join the faculty of the University of Minnesota’s Department of Chemistry in 1927. By the time of his retirement from the University in 1962, he had published approximately 800 papers. He continued to publish approximately 150 more papers until his health failed. His research, covering approximately a dozen areas of chemistry, was recognized by many medals and memberships in learned societies throughout the world, including the National Academy of Sciences and the Nichols Medal of the American Chemical Society. Best known to the general public is his work on synthetic rubber. During World War II, the government established a comprehensive research program at major industrial companies and several universities, including Minnesota. Kolthoff quickly assembled a large research group and made major contributions to the program. Many of Kolthoff’s graduate students went on to successful careers in industry and academic life and, in turn, trained many more. In 1982, it was estimated that approximately 1,100 Ph.D. holders could trace their scientific roots to Kolthoff. When the American Chemical Society inaugurated an award for excellence in 1983, he was the first recipient.
Dr. Olugbeminiyi Fadeyi
Thursday, Feb. 4, 2021, 11:15 a.m. through Thursday, Feb. 4, 2021, 12:15 p.m.
Mapping Cell-Cell Interactions in Tumor Microenvironment via Photocatalytic Proximity Labeling
Membrane proteins play essential roles in an extensive range of cellular functions. One notable example is the surface interaction between immunomodulatory receptors (IMRs) to initiate and regulate immune responses. The success in modulating these interactions with checkpoint inhibitors such as anti-PDL1/CTLA4 demonstrates the tremendous therapeutic value in understanding how IMRs interact and the impact of neighboring proteins on modulating IMR function. Protein proximity labeling represents a powerful approach for the unbiased assessment of protein-protein interactions or bystander proteins with effector function on the cell surface. A number of enzyme-based proximity labeling strategies have been developed over the last decade that either generate a reactive labeling species in proximity to the protein of interest, or physically "stamp" neighboring proteins. The success of these approaches has led to the consideration of non-enzyme based methods that are smaller in size, can be temporally controlled, and/or can avoid harsh treatment conditions. This talk will describe the development of novel photocatalytic-based proximity labeling approaches whereby protein residues are labeled in the presence of visible light and a photocatalyst. Applications of this technology on the cell surface and within cell interaction environments will also be showcased.
Olugbeminiyi (Niyi) Fadeyi
Originally from Nigeria, Niyi completed his B.Sc in Chemistry at Obafemi Awolowo University, Nigeria under the direction of Professor Craig Obafemi. Following his undergraduate studies, he worked for GlaxoSmithKline, Nigeria as a scientist and later moved to the US for graduate studies. He obtained his Masters in 2007 from Tennessee State University, under the direction of Prof. Cosmas Okoro.
He continued on to the doctoral program at Vanderbilt University, where under the mentorship of Prof. Craig Lindsley, his research focused on developing new synthetic methodologies and application to towards natural products synthesis. In 2011, Niyi obtained his doctorate degree and joined Prof. Matthew Shair’s laboratories at the department of chemistry and chemical biology, Harvard University as a UNCF/Merck postdoctoral fellow. He joined Pfizer Inc., Groton in 2014, where he was a Principal Scientist with the Inflammation & Immunology-Rare Diseases division in World Wide Medicinal Chemistry. After 3 years at Pfizer, he joined Merck Exploratory Science Center as a Molecular Invention Scientist where he is developing a platform that integrates chemistry and biology to study novel mechanistic basis of human diseases to develop new therapeutics.
Professor Richmond Sarpong
Thursday, Feb. 4, 2021, 9:45 a.m. through Thursday, Feb. 4, 2021, 11 a.m.
Professor Richmond Sarpong
Department of Chemistry
University of California, Berkeley
Host: Professor Thomas Hoye
Break-it-to-Make-it Strategies for Chemical Synthesis Inspired by Complex Natural Products
Natural products continue to inspire and serve as the basis of new medicines. They also provide intricate problems that expose limitations in the strategies and methods employed in chemical synthesis. Several strategies and methods that have been developed in our laboratory and applied to the syntheses of architecturally complex diterpenoid alkaloids, indole alkaloids, and several Lycopodium alkaloids, will be discussed. In addition, new ways to employ C–C bond cleavage in synthesis will be presented (i.e., break-it-to-make-it strategies).

Professor Sarpong
Richmond Sarpong is a professor of chemistry at the University of California, Berkeley, where he and his group specializes in synthetic organic chemistry. He became interested in chemistry after seeing, firsthand, the effectiveness of the drug ivermectin in combating river blindness during his childhood in Ghana, West Africa. He described his influences and inspirations in a TEDxBerkeley talk in 2015 (Face of Disease in Sub-Saharan Africa). Professor Sarpong completed his undergraduate studies at Macalester College in St. Paul, MN, with Professor Rebecca C. Hoye and his graduate work was carried out at Princeton. He conducted postdoctoral studies at the California Institute of Technology.
At Berkeley, Professor Sarpong’s laboratory focuses on the synthesis of bioactive complex organic molecules, with a particular focus on secondary metabolites that come from marine or terrestrial flora and fauna. These natural products continue to serve as the inspiration for new medicines. It is Sarpong’s hope that through the work in his laboratory, he and his coworkers will uncover methods and strategies for synthesis that may contribute to more efficient ways to prepare bioactive compounds that may inspire new medicines.
Jeannette Brown Lectureship
Alumna Jeannette Brown’s pioneering legacy includes being a talented chemist in the pharmaceutical industry for 25 years, author, historian, and tireless leader and advocate for the inclusion and advancement of African American women in chemistry-related professional pursuits and careers. Brown is the first African American to receive a degree from the Department of Chemistry's graduate program, earning her master's degree in 1958. She is a former faculty associate in the department of Pre-College Programs at the New Jersey Institute of Technology. For 25 years, she worked as a research chemist at Merck. She is the author of two books, "African American Women Chemists" and "African American Women Chemists in the Modern Era." She is a Société de Chimie Industrielle (American Section) Fellow of the Chemical Heritage Foundation (2004), and is a member of the first class of American Chemical Society (ACS) Fellows (2009). For her distinguished service to professionalism, she received the Henry Hill Award from the ACS Division of Professional Relations in 2020. For her work as a mentor to minority students and science education advocacy, she was elected to the Hunter College Hall of Fame in 1991; was honored by the University of Minnesota with an Outstanding Achievement Award in 2005; and received the ACS national award for Encouraging Disadvantaged Students into Careers in the Chemical Sciences in 2005.
Professor Richmond Sarpong
Wednesday, Feb. 3, 2021, 10 a.m. through Wednesday, Feb. 3, 2021, 11:30 a.m.
Zoom (or use the ZOOM link below in the full schedule)
Professor Richmond Sarpong
Department of Chemistry
University of California, Berkeley
Host: Professor Thomas Hoye
A Life Shaped by Diseases and Medicine in Sub-Saharan Africa
Richmond Sarpong is a professor of chemistry at the University of California, Berkeley, where he and his group specialize in synthetic organic chemistry. In this lecture, Sarpong will describe the cultural, environmental, and personal influences of mentors that led him to a career as a faculty member in the chemical sciences. He became interested in chemistry after seeing, firsthand, the effectiveness of the drug Ivermectin in combating river blindness during his childhood in Ghana, West Africa. He described his influences and inspirations in a TEDxBerkeley talk in 2015, Face of Disease in Sub-Saharan Africa.
Professor Sarpong
At Berkeley, Professor Sarpong’s laboratory focuses on the synthesis of bioactive complex organic molecules, with a particular focus on secondary metabolites that come from marine or terrestrial flora and fauna. These natural products continue to serve as the inspiration for new medicines. It is Sarpong’s hope that through the work in his laboratory, he and his coworkers will uncover methods and strategies for synthesis that may contribute to more efficient ways to prepare bioactive compounds that may inspire new medicines.
Professor Sarpong completed his undergraduate studies at Macalester College in St. Paul, MN, and carried out undergraduate studies with Professor Rebecca Hoye. He earned his doctorate from Princeton, which was followed by post-doctoral studies at Caltech.
Jeannette Brown Lectureship
Alumna Jeannette Brown’s pioneering legacy includes being a talented chemist in the pharmaceutical industry for 25 years, author, historian, and tireless leader and advocate for the inclusion and advancement of African American women in chemistry-related professional pursuits and careers. Brown is the first African American to receive a degree from the Department of Chemistry's graduate program, earning her master's degree in 1958. She is a former faculty associate in the department of Pre-College Programs at the New Jersey Institute of Technology. For 25 years, she worked as a research chemist at Merck. She is the author of two books, "African American Women Chemists" and "African American Women Chemists in the Modern Era." She is a Société de Chimie Industrielle (American Section) Fellow of the Chemical Heritage Foundation (2004), and is a member of the first class of American Chemical Society (ACS) Fellows (2009). For her distinguished service to professionalism, she received the Henry Hill Award from the ACS Division of Professional Relations in 2020. For her work as a mentor to minority students and science education advocacy, she was elected to the Hunter College Hall of Fame in 1991; was honored by the University of Minnesota with an Outstanding Achievement Award in 2005; and received the ACS national award for Encouraging Disadvantaged Students into Careers in the Chemical Sciences in 2005.