Carboxylic sulfuric anhydrides featured in Journal of Physical Chemistry

The Journal of Physical Chemistry A published a feature article from Professor Kenneth Leopold's group on “Carboxylic Sulfuric Anhydrides." The paper presents new spectroscopic data on propiolic sulfuric anhydride and reviews results for a series of carboxylic sulfuric anhydrides (CSAs) studied by this group over the past four years. It also reports new statistical thermodynamic calculations of the equilibrium constants for their formation. The authors of the article are C.J. Smith, Anna K. Huff, Rebecca M. Ward (née Mackenzie), and Kenneth R. Leopold. The paper was also featured as an ACS Editors’ Choice article.
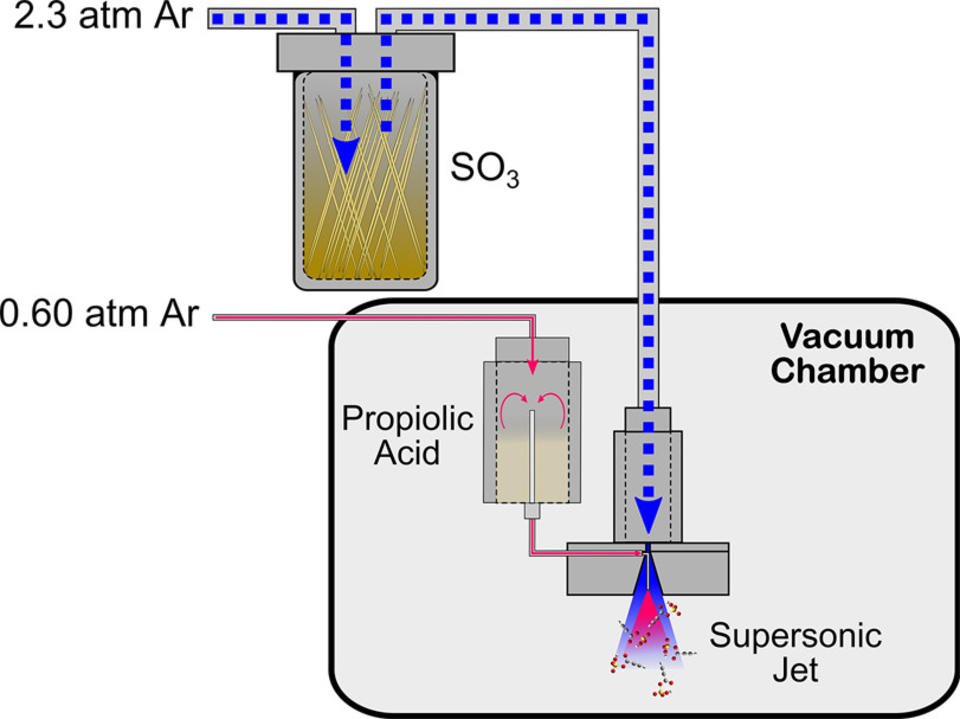
The underlying discovery is that sulfur trioxide (SO3) readily reacts with carboxylic acids to form carboxylic sulfuric anhydrides, a class of compounds not widely recognized in the literature. The gaseous reactants, entrained in an argon carrier gas, are expanded in a supersonic jet into vacuum (Figure 1). The resulting gas plume contains anhydride molecules at a nominal temperature of ~2 K, where they can then be conveniently probed by microwave spectroscopy.
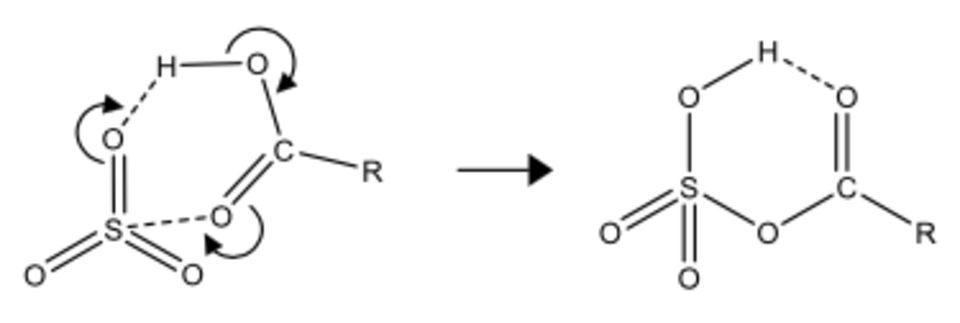
The formation is clearly fast, as it must take place during collisional phase of the expansion, which lasts only several tens of microseconds. The proposed mechanism is shown in Figure 2. A portion of the spectrum observed for the formic acid derivative (R = H) between 9 and 15 GHz is seen in the cover art above. Accompanying theoretical calculations indicate that the zero-point corrected activation barriers relative to the putative precursor complexes are very close to zero and in some cases negative, consistent with the facile formation of these species under supersonic jet conditions. Calculated equilibrium constants for the reaction RCOOH(g) + SO3(g) ⇄ RCOOSO2OH(g) for several representative R groups range from 104 atm-1 for formic acid at 288 K to over 1011 atm-1 for benzoic acid at 217 K.
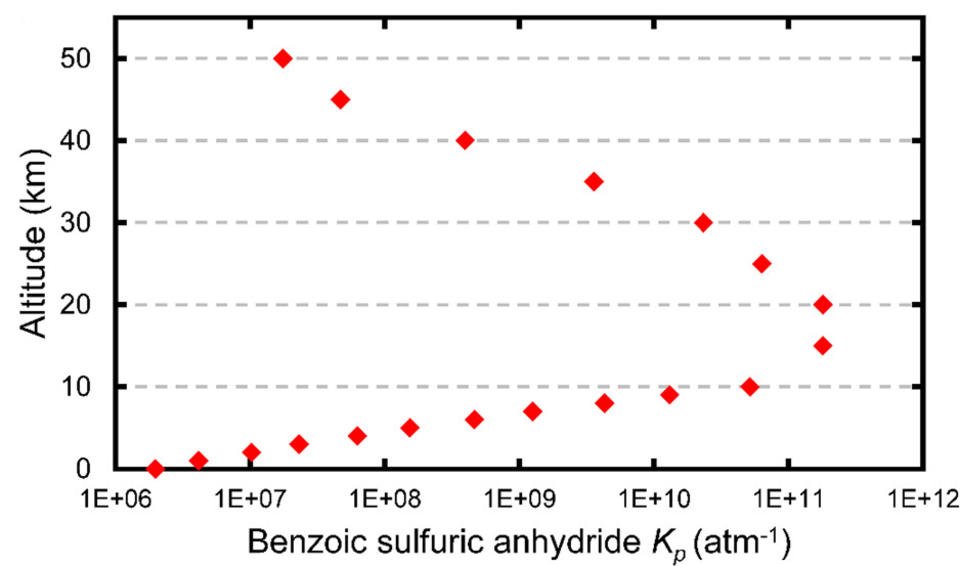
SO3 and carboxylic acids are important trace gases in the atmosphere, and both are intimately related to aerosol formation and composition. If CSAs form in the atmosphere, their hydrolysis in clusters or pre-existing aqueous droplets could provide a mechanism for shuttling low molecular weight organic matter in atmospheric aerosol particles. Alternatively, in select circumstances, they could, themselves, seed atmospheric nucleation. Both roles are speculative at this point. The temperature dependence of the CSA formation constants can be translated into an altitude dependence, given a typical atmospheric temperature profile and the results for benzoic sulfuric anhydride are shown in Figure 3. The equilibrium constants reach their maximum value at the tropopause, where the temperature is ~217 K. The results of this study suggest that (i) anhydride concentrations could, under select circumstances, reach levels typically needed to induce nucleation, and (ii) equilibrium concentrations of the anhydrides likely exceed those of the atmospheric sulfuric acid precursors SO3–H2O and SO3–(H2O)2 by several orders of magnitude. However, more work is needed to establish reliable estimates of the concentrations of SO3 and common carboxylic acids at various altitudes, and to create kinetic models applicable to atmospheric conditions, where the equilibrium state is not guaranteed.