Scientists synthesize and characterize, for the first time, actinide 2-metalla-biphenylene compounds
Scientists from the Department of Chemistry and the Los Alamos National Laboratory have synthesized and characterized the first 2-metallabiphenylene compounds, advancing the knowledge of actinide systems that can show unique spectroscopies and reactivities.
Aromaticity and antiaromaticity, as defined by Hückel’s rule, are key ideas taught in freshman organic chemistry. Aromaticity is a property of ring-shaped flat structures where there is a ring of resonance bonds that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Hückel’s rule, developed by German chemist and physicist Erich Hückel in 1931, is used to determine if a flat ring molecule has aromatic properties. Antiaromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of delocalised π or lone pair electrons in it.
Both aromaticity and antiaromaticity are exemplified in biphenylene, which is a molecule that consists of two benzene rings joined by a four-membered ring at its core. Biphenylene contains antiaromatic cyclobutadiene and aromatic benzene rings. The alternating fusion between benzene and cyclobutadiene rings (6–4–6 π-electrons) causes unusual activation of the π- and σ-frameworks, which imparts interesting spectroscopic properties. Biphenylene analogues in which one of the benzene rings has been replaced by a different (4n + 2) π-electron system have so far been associated only with organic compounds.
In the context of displaying new reactivity patterns, a team formed by Jaqueline L. Kiplinger, Ph.D., and colleagues at Los Alamos National Laboratory and Professor Laura Gagliardi and post-doctoral associate Jing Xie, Ph.D., at the University of Minnesota wondered whether the high nodality of the actinide 5f orbitals could enable the formation of a 2-metallabiphenylene complex. Actinides are rare earth metals found in nature that are unstable due to their reactivity. This study resulted in the synthesis and characterization of the first 2-metallabiphenylene compounds.
Reduction of (C5Me5)2AnCl2 (where An is Th or U) with KC8 in the presence of 1,2-bis(phenylethynyl)benzene affords a bright yellow Th-containing and a brown–red U-containing 2-metallabiphenylene complex, (C5Me5)2An(2,5-Ph2-cyclopentadienyl[3,4]cyclobuta-[1,2]benzene). These complexes are the first examples of 2-metallabiphenylenes and also represent the first examples of metal-supported antiaromatic systems in which the metal does not participate directly in the antiaromatic ring.
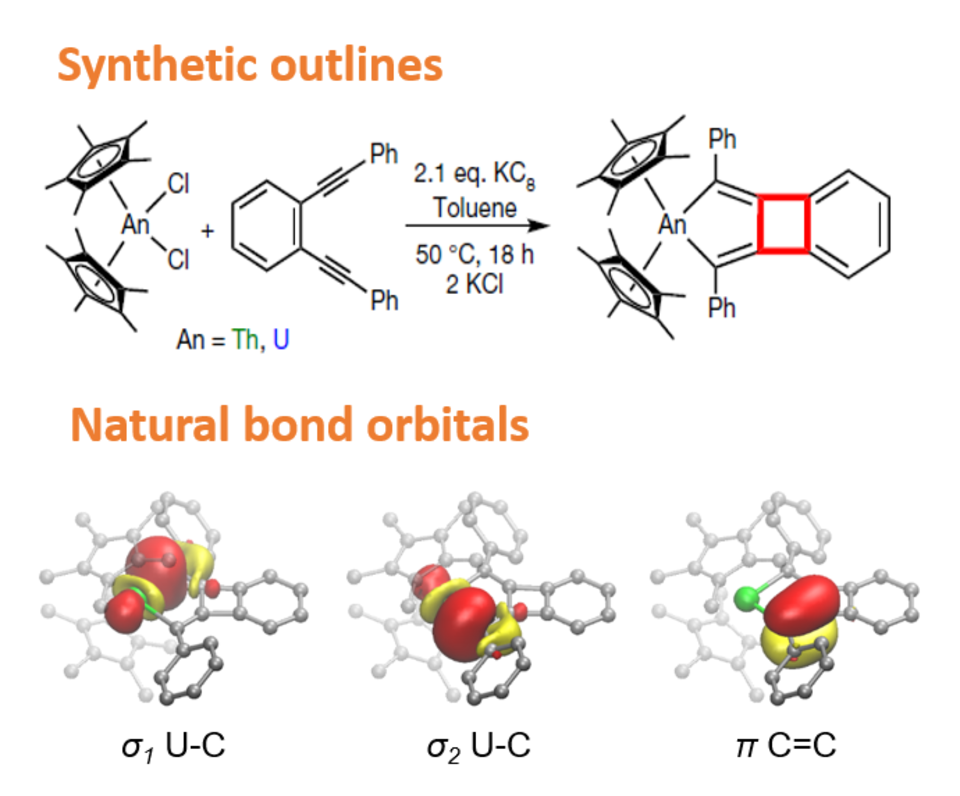
To understand the electronic structure of these species, Xie performed calculations using density functional theory and multireference methods that showed that, while the thorium compound is diamagnetic, the uranium compound has a triplet U(IV) ground state. By performing nucleus independent chemical shift (NICS) calculations, the researchers showed that the four-membered ring is antiaromatic and the six-membered ring is aromatic, resembling biphenylene and its analogues. For the five-membered ring that contains an actinide element, Kohn-Sham molecular orbital analysis and natural bond orbital analysis show that the uranium–metallabiphenylene bonding interaction has substantial covalent character with notable participation of the uranium 5f orbitals in the bonding. A direct comparison was made between experimental UV-Vis spectra and those calculated from time-dependent density functional theory. The researchers showed that the ligand-metal charge transfer of the uranium-complex in the UV region has greater intensity than that of the thorium compound, which can be attributed to the greater degree of metal–metallabiphenylene ligand-bonding interaction.
This work has been published in Nature, "Actinide 2-metallabiphenylenes that satisfy Hückel’s rule" and featured in Chemistry World.
Jing Xie, Ph.D., is currently an assistant professor at the School of Chemistry and Engineering, Beijing Institute of Technology, Beijing, China.
The computational part of this work was funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US DOE through grant USDOE/DESC002183. The calculations were carried out at the Minnesota Supercomputing Institute.