Past Seminars & Events
Professor Sahar Sharifzadeh
Thursday, March 31, 2022, 9:45 a.m. through Thursday, March 31, 2022, 11 a.m.
Professor Sahar Sharifzadeh
Department of Electrical and Computer Engineering
Division of Materials Science and Engineering
Boston University
Host: Professor Renee Frontiera
Understanding the Optoelectronic Properties of 1D Materials from First-Principles Theory
Theory and computation provide important tools for understanding and predicting material properties on the atomic scale, and guiding synthesis and experimentation. In this talk, I will present our recent advances in applying state-of-the-art first-principles computational methods to understand the excited-state electronic properties of 1D-stacked organic assemblies and single walled carbon nanotubes (SWCNTs). In particular, we explore the role that structural complexity, due to the presence of defects and vibrations, plays on electronic and optical excitations in these materials, and develop physical intuition to explain these phenomena.
Sahar Sharifzadeh
Dr. Sahar Sharifzadeh is an Associate Professor at Boston University. She obtained her PhD from Princeton University, working under the guidance of Prof. Emily Carter, and subsequently joined the Molecular Foundry at Lawrence Berkeley National Laboratory as a postdoctoral fellow and project scientist in the group of Dr. Jeffrey Neaton. She joined Boston University in 2014 as an Assistant Professor. Prof. Sharifzadeh was awarded the Department of Energy Early Career Award in 2017, the National Science Foundation Early Career Award in 2019, and the Boston University College of Engineering Early Career Award in 2019. Her research focuses on first-principles computational modelling of materials.
Professor Janet Morrow
Tuesday, March 29, 2022, 9:45 a.m. through Tuesday, March 29, 2022, 11 a.m.
331 Smith Hall
Zoom Link
Professor Janet Morrow
UB Distinguished Professor
Larkin Professor
Department of Chemistry
College of Arts and Sciences
University of Buffalo
Host: Professor Valerie Pierre
Macrocycles, Cages and Liposome-based Cobalt and Iron Complexes as Magnetic Resonance Imaging Probes
MRI probes or contrast agents contain paramagnetic metal ion complexes that produce contrast through changes in proton relaxation times or water proton shift. Research in our laboratory is focused on transition metal complexes as 1) alternatives to Gd(III) based contrast agents, 2) probes that are responsive to the biological environment and 3) as agents that accumulate in tumors for drug delivery. Macrocyclic ligands are central to our research through modulating the spin and oxidation states of cobalt and iron complexes in aqueous solutions. For example, macrocyclic complexes of high spin Co(II) produce highly shifted water protons and can be loaded into liposomes and/or incorporated into the bilayer to produce chemical exchange saturation transfer (lipoCEST) agents. These liposomal agents are responsive to redox or pH changes in solution as detected by a change in the asymmetry of the z-spectrum. High spin Fe(III) macrocyclic complexes show enhanced water proton T1 relaxation as potential Gd(III) alternatives. Incorporation of Fe(III) complexes into liposomes produces T1 agents for tracking the delivery of intraliposomal contents to murine tumors. Finally Fe(III) centers can be linked together to form self-assembled cages that behave as blood pool agents and accumulate in murine tumor models.
Janet Morrow
Janet Morrow received her B.S. in Chemistry at the University of California, Santa Barbara and her Ph.D. in Inorganic Chemistry at the University of North Carolina, Chapel Hill. From there she did postdoctoral work for three years, first at the University Bordeaux, France on a NSF postdoctoral fellowship and then at the University of California, San Diego. She started her independent career at the University at Buffalo, the State University of New York in 1988, and moved up through the ranks to her current position as SUNY Distinguished Professor and Larkin Chair. Her research interests are in the field of bioinorganic chemistry with a current focus on transition metal ion complexes as probes for biomedical imaging. Her research contributions have been recognized with several awards, including the American Chemical Society Schoellkopf Medal, UB Exceptional Scholar, Alfred P. Sloan fellowship, NIH First award and the NSF Special Award for Creativity. She is currently an associate editor of the ACS journal, Inorganic Chemistry. She is an elected fellow of the American Association for the Advancement of Science (AAAS).
DEI Seminar: Crystal Lee-Thao
Monday, March 28, 2022, 4 p.m. through Monday, March 28, 2022, 5 p.m.
231 Smith Hall
Zoom Link
Crystal Lee-Thao
Dept. of Organizational Leadership, Policy, & Development
University of Minnesota
Host: Chemistry D&I Grad Student Training Working Group
Engagement Means...: Community College Students’ Understandings of Engagement
Research in higher education frequently report low levels of student engagement among community college students. What is commonly overlooked in these discussions are the students’ own understandings of engagement and how their understanding of the concept is connected to their student success. This qualitative study involved interviewing 11 students at an urban community college in the Midwest to better understand their engagement experiences and practices. Using Bronfenbrenner’s ecological systems theory as a conceptual framework, the analysis of experiences revealed that participants conceptualized student engagement in three major ways: 1) compassion 2) academic motivation and 3) activism. Additionally, these three themes existed and interacted within all of Bronfenbrenner’s ecological systems. Implications for practice and research, as well as an informal model of community college student engagement, are included.
Crystal Lee-Thao
Crystal Lee-Thao earned her B.A. in Communications and Rhetorical Studies, at the University of Wisconsin – Madison, and a M.A. in Higher Education from the University of Minnesota - Twin Cities. She is currently a Ph.D. student in the Department of Organizational Leadership, Policy, & Development at the University of Minnesota and the Assistant Director for the Louis Stokes North Star STEM Alliance.
Professor Angela M. Gronenborn
Thursday, March 24, 2022, 9:45 a.m. through Thursday, March 24, 2022, 11 a.m.
Professor Angela M. Gronenborn
Department of Structural Biology and Pittsburgh Center for HIV-Protein Interaction
University of Pittsburgh School of Medicine
Host: Professor William Pomerantz
The awesome power of fluorine NMR
19F NMR is a powerful and versatile tool to study protein structure and protein ligand interactions due to the favorable NMR characteristics of the 19F atom, its small size and absence in naturally occurring biomolecules. 19F atoms can be introduced readily into proteins and ligands, permitting to use them as ‘beacons’ to study interactions by NMR. Both, ligand and protein resonances can be exploited for this purpose. I will discuss several applications, involving 19F-modified proteins and 19F-containing ligands, demonstrating the awesome power of 19F NMR.
Angela M. Gronenborn
Dr. Angela Gronenborn heads the Department of Structural Biology at the University of Pittsburgh School of Medicine and holds the UPMC Rosalind Franklin Chair. Throughout her career, Dr. Gronenborn was involved in developing NMR methodology for structure determination of biological macromolecules. In the area of HIV research, Dr. Gronenborn directs the Pittsburgh Center for HIV Protein Interactions (PCHPI). In recent years, Dr. Gronenborn has focused on extending the application of NMR to the study of complex systems, in particular developing fluorine NMR approaches. Dr. Gronenborn is an elected Fellow of the Royal Society of Chemistry, U.K., the American Association for the Advancement of Science and the International Society of Magnetic Resonance. She is a member of the Washington and New York Academies of Sciences and was elected to the National Academy of Sciences, the Norwegian Academy of Arts and Letters, the German National Academy of Sciences and the American Academy of Arts & Sciences.
Professor Davit Potoyan
Friday, March 18, 2022, 9:30 a.m. through Friday, March 18, 2022, 10:30 a.m.
117/119 Smith Hall and Zoom
Professor Davit Potoyan
Department of Chemistry
Iowa State University
Host: Professor Ilja Siepmann
Multi-scale computational studies of assembly, regulation, and phase separation in the cell nucleus
Cells of higher organisms are known for the hierarchical self-organization of their genomes, proteome, and associated biochemical reactions. Uncovering the underlying driving forces for cellular self-organization is a topic of significant importance in biosciences. Recent experiments have revealed the ubiquitous presence of nano and microscale membranless compartments in the nuclei of cells, generated through liquid-liquid phase separation of protein and nucleic acid components. Due to the heterogeneous and non-equilibrium environment, nuclear compartmentalization's thermodynamic and kinetic aspects are challenging to study both in vivo and in silico.
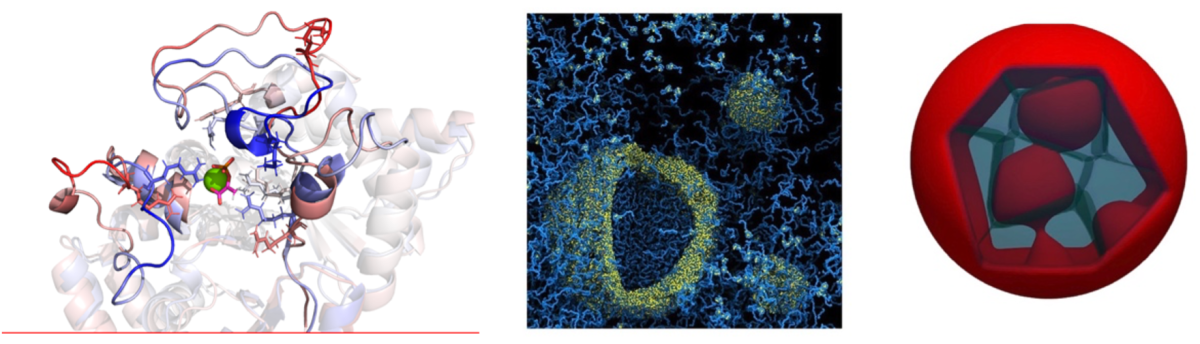
Our group is developing and applying multi-scale computational models that use atomistic, coarse-grained, and phase-field techniques to study nuclear compartmentalization at different scales, in and out of equilibrium. In the talk, we will present a selection of recent results on protein-RNA phase transitions, mesoscale nuclear dynamics of chromatin phase separation, and detailed models of biomolecular condensates based on bioinformatics and atomistic simulations.
Davit Potoyan
Davit Potoyan received his Ph.D. in Chemical Physics at the University of Maryland-College Park in 2012. He spent the next few years training as a postdoctoral fellow in the Center for Theoretical Biological Physics at Rice University developing theoretical and computational models for studying gene-regulatory networks and transcription factor DNA assembly. In 2017 Davit joined the Iowa State as a Caldwell Assistant Professor of Chemistry and currently holds a courtesy faculty appointment in BBMB and BCB programs. The research field of Professor Potoyan is in computational biophysics broadly defined. His group is using multi-scale computational tools rooted in statistical physics, bioinformatics, machine learning, and data analytics to work on various biologically motivated problems. Some of the active areas of research in the group include the condensation of disordered proteins and nucleic acids, enzyme dynamics, chromatin organization, and genetic regulatory networks.
Professor Robert J. Gilliard, Jr.
Thursday, March 17, 2022, 9:45 a.m. through Thursday, March 17, 2022, 11 a.m.
Professor Robert J. Gilliard, Jr.
Department of Chemistry
University of Virginia
Host: Professor Ian Tonks
Beryllium, Boron, and Bismuth: From Fundamental Redox Chemistry to Luminescent and Thermochromic Materials
Research efforts in the Gilliard laboratory span diverse areas of chemical synthesis. While the redox chemistry of transition metals is established, the development of main-group element-mediated redox cycles remain a major challenge. This is in part due to inherent differences in electronic structure and the highly reactive nature of main-group elements in low oxidation states. Thus, we have been interested in the design and isolation of low-valent and cationic main-group compounds that impact energy-relevant molecular transformations, including reactions with small molecules (e.g., carbon dioxide, dihydrogen). Our work has mostly centered around establishing bonding and reactivity trends among the alkaline earth metals (Be, Mg) and heavy pnictogens (Sb, Bi). Recently, we have begun to study heterocycles “doped” with boron for the development of new π-electron materials with unusual bonding and photophysical properties. This has led to the first examples of pyrene-fused N-heterocyclic boranes, thermochromic and thermoluminescent borafluorenes, and stable boracyclic radicals. Our primary goal has been to isolate molecules in rare electronic states and to provide a link between structure and function. This presentation will highlight our most recent results in these research areas.
Robert J. Gilliard, Jr.
Prof. Gilliard is a native of Hartsville, South Carolina. He obtained his bachelor’s degree in chemistry at Clemson University where he was an undergraduate researcher in the laboratory of Prof. Rhett C. Smith. He earned his doctorate in chemistry at The University of Georgia with Prof. Gregory H. Robinson. Gilliard was a Merck Postdoctoral Fellow and a Ford Foundation Postdoctoral Fellow where he completed his studies working jointly at the Swiss Federal Institute of Technology (ETH Zürich) with Prof. Hansj rg Grützmacher and at Case Western Reserve University with Prof. John Protasiewicz. Gilliard joined the faculty at the University of Virginia as an Assistant Professor of Chemistry in the Fall of 2017. He has received several awards and honors. Recent honors include: named to Forbes Magazine 30 under 30 list in Science, Inorganic Chemistry and Chemistry-A European Journal Emerging Investigator, Chemical and Engineering News Talented 12 Scholar, Scialog Collaborative Innovation Award, National Science Foundation CAREER Award, Alfred P. Sloan Research Fellow, 3M Non-Tenured Faculty Award, Organometallics Distinguished Author Award, Beckman Young Investigator Award, Packard Fellowship. He also serves on the editorial advisory board for Chemical Communications, Chem Catalysis, Inorganic Chemistry, and Angewandte Chemie.
Professor Elizabeth Elacqua
Tuesday, March 15, 2022, 9:45 a.m. through Tuesday, March 15, 2022, 11 a.m.
Professor Elizabeth Elacqua
Department of Chemistry
Pennsylvania State University
Host: Professor Marc Hillmyer
Merging Organic Synthetic and Polymer Chemistry: Toward Accelerated Catalysis, Sequence Definition, and Architecturally-Diverse Sp3-Enriched Polymers
Efforts to develop synthetic methods that achieve robust materials (e.g., sequenced organic electronics, polymerizable renewable feedstocks, and/or sustainable cooperative catalysis) have generated a need to engineer strategies that merge organic synthesis and polymer chemistry to address grand challenges. Our group’s research is inspired by Nature and founded on using polymer chemistry to address shortcomings in organic synthesis, and using organic chemistry to confront challenges in polymer synthesis. This talk will detail our group’s recent efforts at this interdisciplinary interface. We will first discuss our homogeneous polymer catalysts that are visible-light activated and feature significant rate acceleration in cooperative organic photoredox catalysis, ascribed to more efficient single-electron transfer. Our approach deviates from conventional methods, and tackles diffusion-limited cooperative catalysis, while enabling enhanced reactivity under polymer confinement. Second, we will disclose the synthesis of sp3-hybridized 1D carbon-based polymers from simple petroleum-based or biomass-derived sp2 feedstocks under pressure. In these studies, we have uncovered new robust materials from abundant aromatics (e.g., furan, phenol, pentafluorophenol) that are theorized to possess high tensile strength and chemical versatility.
Elizabeth Elacqua
Beth was born and raised in upstate New York, and received her B.S. Degree in Chemistry and Biology from LeMoyne College (Syracuse, NY) in 2006. There, she conducted research focused on the synthesis of porphyrins as nitrogenase mimics, as well as on the total synthesis of natural products such as polyphenolic stilbenoids. After spending a year at SUNY College of Environmental Science and Forestry working in the lab of Dr. Israel Cabasso at the Michael Szwarc Polymer Research Institute, she ventured to the University of Iowa for graduate school. At Iowa, Beth worked at the interface of solid-state chemistry, supramolecular chemistry, organic synthesis, and crystal engineering in the research group of Leonard R. MacGillivray, and received her Ph.D. in 2012. After graduation, Beth headed back to the great state of New York where she started as a Postdoctoral Research Associate at New York University working alongside Marcus Weck. At NYU, Beth utilized her background in supramolecular chemistry and crystal engineering to work on developing β-sheet-mimicking telechelic polymers for self-assembly, as well as designing reversibly-assembling colloidal matter.
Professor Amar Flood
Thursday, March 3, 2022, 9:45 a.m. through Thursday, March 3, 2022, 11 a.m.
This seminar will be presented 'in person' and live-streamed
331 Smith Hall
Zoom Link
Professor Amar Flood
Department of Chemistry
Indiana University
Host: Professor Valerie Pierre
Anion Recognition and Hierarchical Assembly
Ions are intimately related to the sustainable and technological development of our society, which has helped motivate creation of synthetic receptors to manage cations and anions. Of these, cations have enjoyed the lion’s share of our attention ever since Werner’s Nobel in 1913 recognized the reliability of their coordination chemistry. Anions have barely had a look in. These negative beasties are large, diffuse and difficult to pin down. Yet they can no longer be ignored. Their roles are diverse and span from the use of dihydrogen-phosphate (H2PO4–) in fertilizer through to hexafluoro-phosphate (PF6−) used as the workhorse electrolyte in Li-ion batteries. This talk will cover recent works tackling these and other anions with shape-persistent and shape-dynamic receptors in the form of cyanostar macrocycles and triazole-based macrocycles, cages and foldamers. Along the way, the interplay between receptor and anion has grown more reliable, whether by design or discovery. This upgrade in status now allows us to learn the rules governing how anions can be used in self-assembly synthesis to control the structures and functions of advanced materials from supramolecular polymers to predictably fluorescent solids we call SMILES.
Amar Flood
Amar Flood was educated at Otago University, New Zealand (BSc Hons 1st, 1996; PhD 2001) under the supervision of Keith C. Gordon. He joined the group of Sir Fraser Stoddart (2002) at UCLA as a postdoctoral scholar conducting research on interlocked molecules and molecular switches. He started at Indiana University in 2005 as an Assistant Professor, was promoted to Associate Professor in 2011, named the James F. Jackson Associate Professor in 2014, and was promoted to full Professor and named the Waterman Professor in 2015. He was Director of Graduate Studies from 2013-2019. He conducts research in four areas: (i) Anion recognition with CH hydrogen bonds. (ii) Molecular switches, both independently and with collaborators. (iii) Ultrabright fluorescent materials by design. (iv) Applications of anion recognition. He has co-organized three international symposia, chaired multiple scientific symposia including NSF and GRC sponsored meetings, is currently funded by the NSF and DOE and he is a Camille Dreyfus Teacher-Scholar.
Professor Michelle Arkin
Tuesday, March 1, 2022, 9:45 a.m. through Tuesday, March 1, 2022, 11 a.m.
Professor and Department Chair Michelle Arkin
Department of Pharmaceutical Chemistry
University of California San Francisco
Host: Professor Ambika Bhagi-Damodaran
Site-directed drug discovery
Michelle Arkin’s lab develops methods and molecules to target currently ‘undruggable targets’ like proteases and protein-protein interactions. This presentation will describe how the lab uses a fragment-based approach called disulfide tethering to discover chemical probes with new mechanisms of action for these two protein classes. Michelle also co-Directs the UCSF Small Molecule Discovery Center, which collaborates on chemical biology and drug discovery research.
Michelle Arkin
Michelle Arkin is professor and chair of Department of Pharmaceutical Chemistry at the University of California, San Francisco. Michelle’s lab develops innovative approaches to screen for chemical tools and drug leads, using biophysical approaches like fragment-based drug discovery and biological approaches including high-content imaging with primary cells and organisms. Michelle is co-Director of the Small Molecule Discovery Center, a collaborative research and core lab that includes a high-throughput screening facility and medicinal chemistry.
Emily Pelton
Thursday, Feb. 24, 2022, 4 p.m. through Thursday, Feb. 24, 2022, 6 p.m.
331 Smith Hall
Zoom Link
Emily Pelton
Senior Lecturer
University of Minnesota
Host: Professor Michelle Driessen
General Chemistry Area Candidate
A pH buffer solution is an aqueous solution that contains a weak acid and its conjugate base (or weak base and its conjugate acid). When small amounts of acid or base are added, the pH of the solution changes very little. Buffer solutions provide a way to keep pH nearly constant in a wide variety of experimental and natural environments. For example, buffer solutions are often used to control pH when studying chemical reactions involving enzymes. In nature, the bicarbonate buffering system is particularly important, especially in the ocean. In this 20 minute mock lecture, Emily will teach how to calculate the pH of a buffer solution after the addition of strong acid and/or base.
Following the mock lecture, Emily will turn to a discussion focussed on her vision for how she might address observations of grade disparities between groups holding a variety of marginalized identities. Data demonstrating these disparities are consistent in introductory STEM courses at many institutions around the country, including the University of Minnesota and including general chemistry. Similar grade disparities are also found in subsequent courses. Emily will describe her vision of how to close gaps observed, with a particular focus on the large lecture modality (i.e., 200-350 students).
Emily Pelton
Emily Pelton earned her BA in 2008 from Gustavus Adolphus College in St. Peter, Minnesota, majoring in ACS Chemistry and minoring in Spanish. She continued her studies in chemistry at the University of Minnesota, earning her MS in 2010 and Ph.D in 2013. Emily has been teaching in the chemistry department at the University of Minnesota since 2013. As a teacher, she focuses on the undergraduate experience in a variety of introductory, general, and organic chemistry courses. Emily’s interests lie in the development and implementation of transformative learning opportunities in the curriculum, especially in terms of assessment strategies, grading structures, and course format. She has had the privilege to teach thousands of students and is continuously examining and improving her teaching and courses to maximize her students’ learning experiences. Emily has won awards from the University of Minnesota’s Center for Educational Innovation to create and implement a hybrid version of Introductory Chemistry, develop online quiz banks for Introductory Chemistry, and develop intentional learning communities in General Chemistry I. In her work to improve student learning outcomes, especially for underrepresented groups, she is currently engaged in the University’s pilot of ECoach, a tailored messaging system designed to help students succeed in large-enrollment gateway STEM courses.